Question
Question: Complete the following equation. A. \(2NaOH + C{l_2} \to \\\ coldanddil \\\ \) B. $C{l_2} + ...
Complete the following equation.
A. 2NaOH+Cl2→ coldanddil
B. C{l_2} + 3{F_2}\xrightarrow{{573K}} \\\
{\text{ (excess)}} \\\
Solution
NaOHis known as sodium hydroxide. It is also called caustic soda. It is an ionic compound which is made of Na+ ion and OH− ion. Cl2 is chlorine gas where two chlorine atoms are bonded to each other. F2 is fluorine gas where two fluorine atoms are bonded to each other.
Complete step by step answer: NaOH in cold and dilute solution is present in dissociated form i.e. Na+ and OH− and some ion exchange reaction will take place. Specifically this is an example of disproportionation reaction.
A disproportionation reaction is a type of redox reaction in which a molecule undergoes oxidation as well as reduction to generate products. The oxidation and reduction products can be found by determining the change in oxidation state of the products compared with the reactant.
In reaction a), the reaction of NaOH and Cl2 will give:
2NaOH+Cl2→NaCl+NaOCl+H2O
The products formed from the reaction of NaOH and Cl2 are sodium chloride salt NaCl and sodium chlorate or sodium hypochlorite NaOCl and water. In order to check whether any reduction or oxidation took place let us analyse the reaction in terms of change of oxidation state.
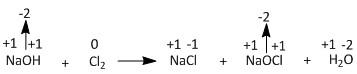
From the above picture it is clear that out of all the elements i.e. Na, O, H and Cl, only Cl is changing the oxidation state from 0 to −1 and +1. The gain of electrons is termed as reduction and the loss of electrons is termed as oxidation. Therefore Cl(0) to Cl(−1) is the reduction of chlorine and Cl(0) to Cl(+1) is the oxidation of chlorine. Hence this is a disproportionation reaction.
In reaction b), the reaction of Cl2 and excess F2 will give:
Cl2+5F2573K2ClF5
The product formed from the reaction of chlorine and fluorine gas is chlorine pentafluoride. The reaction usually takes place at 300∘C. The reaction takes place in a stepwise manner. Actually ClF3 is formed when Cl2 and F2 combine. Then ClF3 combine with F2 to give ClF5.
Cl2+3F2→2ClF3
2ClF3+2F2→2ClF5
In ClF5, the oxidation state of Cl atom is +5 which is very much possible as Cl has vacant 3d orbital. So the electrons from F atom can easily be filled in the vacant 3d orbitals. Thus the valency of Cl atoms is extended and the bonds are formed with five fluorine atoms.
Note: The change in oxidation state has to be monitored to evaluate the type of reaction. Cl atom with its vacant 3d orbitals has the ability to extend its valency and form bonds with multiple atoms.
