Question
Question: Complete the following Diels-Alder reactions. 
Solution
Diels-Alder reaction is a pericyclic reaction. It follows a concerted mechanism. In one single step, all bond breaking and all bond forming takes place. In this reaction, a carbon-carbon triple bond is converted into a double bond. Two new carbon-carbon single bonds are formed. Also, two carbon-carbon double bonds of a conjugated diene are converted into only one double bond.
Complete Step by step answer: In Diels-Alder reaction, an alkene and an alkadiene reacts to form a cyclic product. Usually six member rings are formed as five member and six member rings are stable due to minimum angle and steric strain.
In the Diels-Alder reaction, the conjugated alkadiene is called diene and alkene is called dienophile. Sometimes, instead of alkene, you can also use alkyne as a dienophile.
Diels-Alder reaction, two carbon-carbon pi bonds are broken.
During Diels-Alder reaction, two new carbon-carbon sigma bonds are formed. In other words, during the reaction, two carbon-carbon pi bonds are converted to two carbon-carbon sigma bonds. This is due to concerted bonding between two separate pi electron entities. During the reaction, four pi electrons of conjugated diene and two pi electrons of dienophile shift.
When you react Buta−1,3−diene with Dimethylbut−2−yn−1,4−dioate, you will obtain Dimethyl−4−cyclohexene−1,2−dicarboxylate as the product.
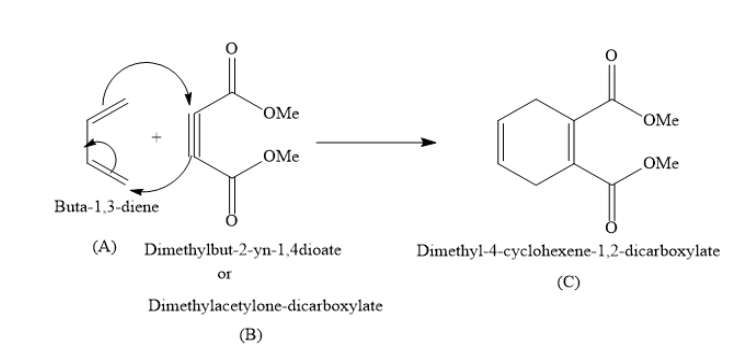
In the above reaction Buta−1,3−diene (A) is called diene and Dimethylbut−2−yn−1,4−dioate (B) is called dienophile. The product Dimethyl−4−cyclohexene−1,2−dicarboxylate is called Diels-Alder adduct.
Note: In Diels-Alder reaction, an alkene (dienophile) and an alkadiene (diene) reacts to form a five or six member ring.
You can use the Diels-Alder reaction for the production of vitamin B6.
You call the reverse reaction as the retro-Diels-Alder reaction. You can use the retro Diels-Alder reaction for industrial manufacture of cyclopentadiene. In this retro Diels Alder reaction, the dimer of cyclopentadiene breaks to give two monomeric units of cyclopentadiene.
