Question
Question: Complete the following chemical reaction a) 
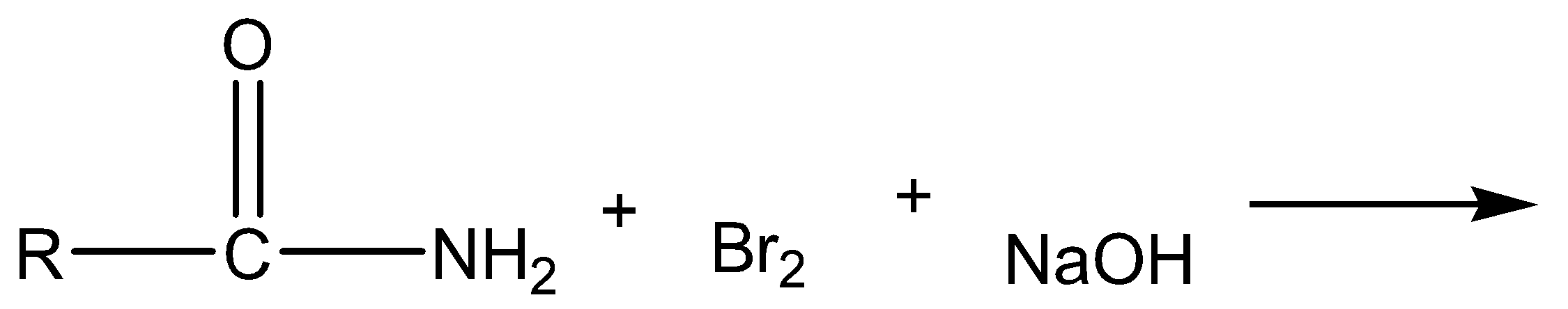
b) R - NH2 + CHI3 + KOH→
c) Convert aniline to sulphanilic acid.
Solution
As we know that in chemistry, equations play a major role. In the reaction three things are main. There are reactants, products and reaction conditions. The reactants are always on the left side of the equation. If more than one reactant in the equation means by using a plus sign. The products are always on the right side of the reaction. Something here also follows. More than one product by using a plus sign. In between reactant products are used arrows to find the reaction direction in the equation.
Complete answer:
We have to know that the balanced chemical equation wise we can predict unknown products in the chemical reaction.
In chemistry, balances the chemical equation means,
We adjust the number moles of reactant and product used to attain an equal number of the same atom in reactant and product in the given chemical reaction.
The given chemical reaction is,
a)
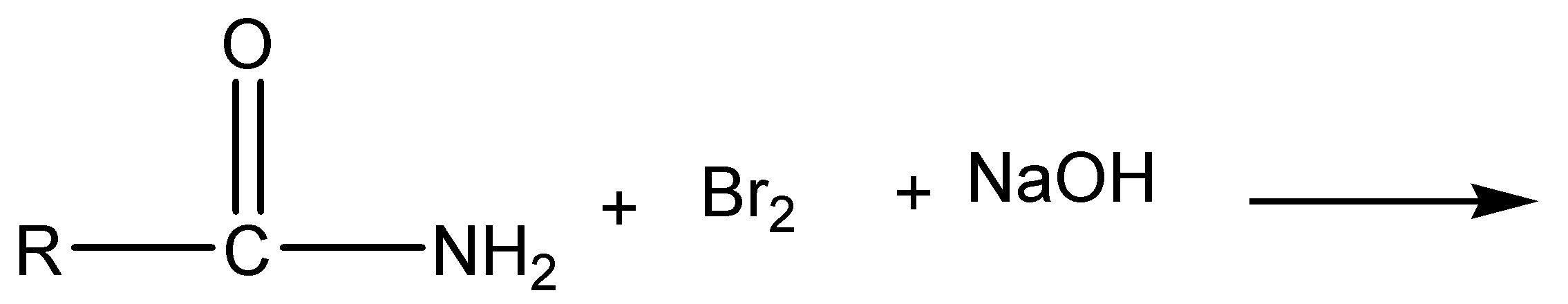
The above reaction is Hoffmann bromamide degradation. In this reaction, amide is reacted with bromine and sodium hydroxide to give off sodium carbonate, sodium bromide and primary amine as products.
The chemical reaction is given below,
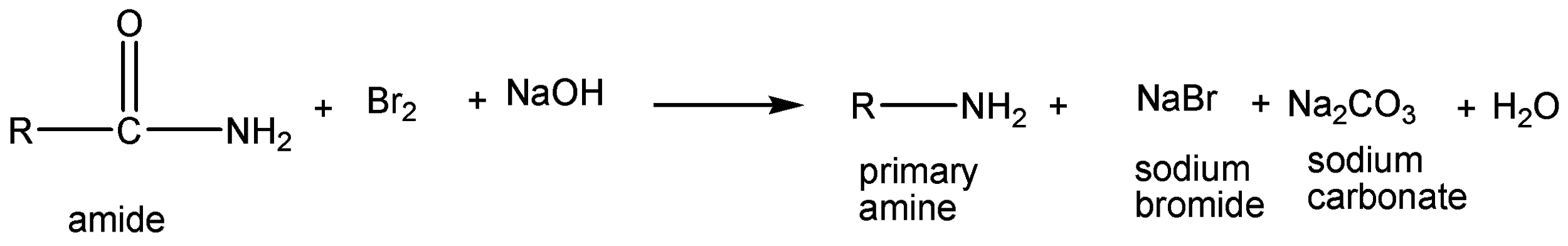
The given chemical reaction is,
b) R - NH2 + CHI3 + KOH→
The above reaction is carbylamine reaction. In this reaction, primary amine is reacted with iodoform and potassium hydroxide to give off potassium iodide and alkyl isocyanide as products.
The chemical reaction is given below,
R - NH2 + CHI3 + 3KOH→R - NC + 3KI + 3H2O
The given chemical reaction is,
c) Convert aniline to sulphanilic acid.
Aniline is reacted with sulphuric acid in pyridine as solvent, to give the product of anilinium hydrogen sulphate. This anilinium hydrogen sulphate is heating with 180∘C - 200∘C to give the product of sulphanilic acid. The commercial name of the product is sulphanilic acid. The IUPAC name of the product is p-aminobenzene sulfonic acid.
The chemical reaction is given below,
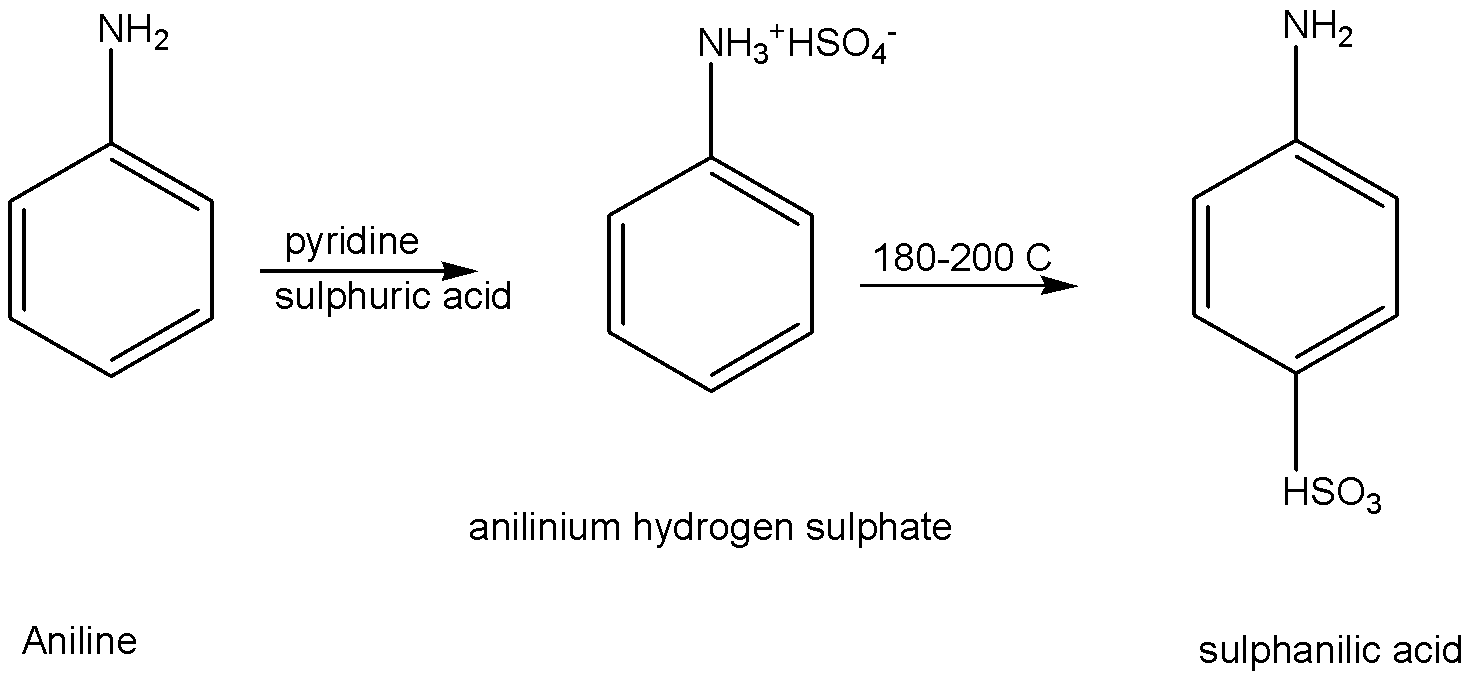
Note:
In balanced chemical reaction wise, we predict the side product of the reaction. In some cases we are not able to attain the balance by changing the mole. In that case we use the number of countable ions in the product and reactant side of the chemical reaction. In chemistry, redox reaction is one of the types of major reactions. In this redox reaction two methods are used to attain the balanced chemical reaction. There are ion-electron methods and the oxidation number method.
