Question
Question: Complete reduction of benzene diazonium chloride with \[Zn/HCl\] gives: A. azobenzene B. phenylh...
Complete reduction of benzene diazonium chloride with Zn/HCl gives:
A. azobenzene
B. phenylhydrazine
C. hydrazobenzene
D. aniline
Solution
Whenever substances like zinc or hydrochloric acid are given in the question, then it reacts as a reducing agent. So, the final product of the reaction will be a reduced form of benzene diazonium chloride.
Complete step by step solution:
Benzene diazonium chloride is actually a benzene ring, which has a diazonium group attached to it. In the diazonium group, the nitrogen attached to the chlorine has a negative charge, whereas the nitrogen attached with the other nitrogen and the benzene ring carries a positive charge. Zinc and HCl are very good reducing agents. They strongly reduce other compounds and get oxidised themselves. So, when benzene diazonium chloride reacts with zinc and hydrochloric acid, the latter part reduces the diazo group and adds hydrogen to it. We obtain phenyl hydrazine when taken in small amounts. But as it is a strong reducing agent, it will reduce the compound further and form aniline. Since aniline is formed as the major product.
So option D is the correct answer.
Let us see the reaction:
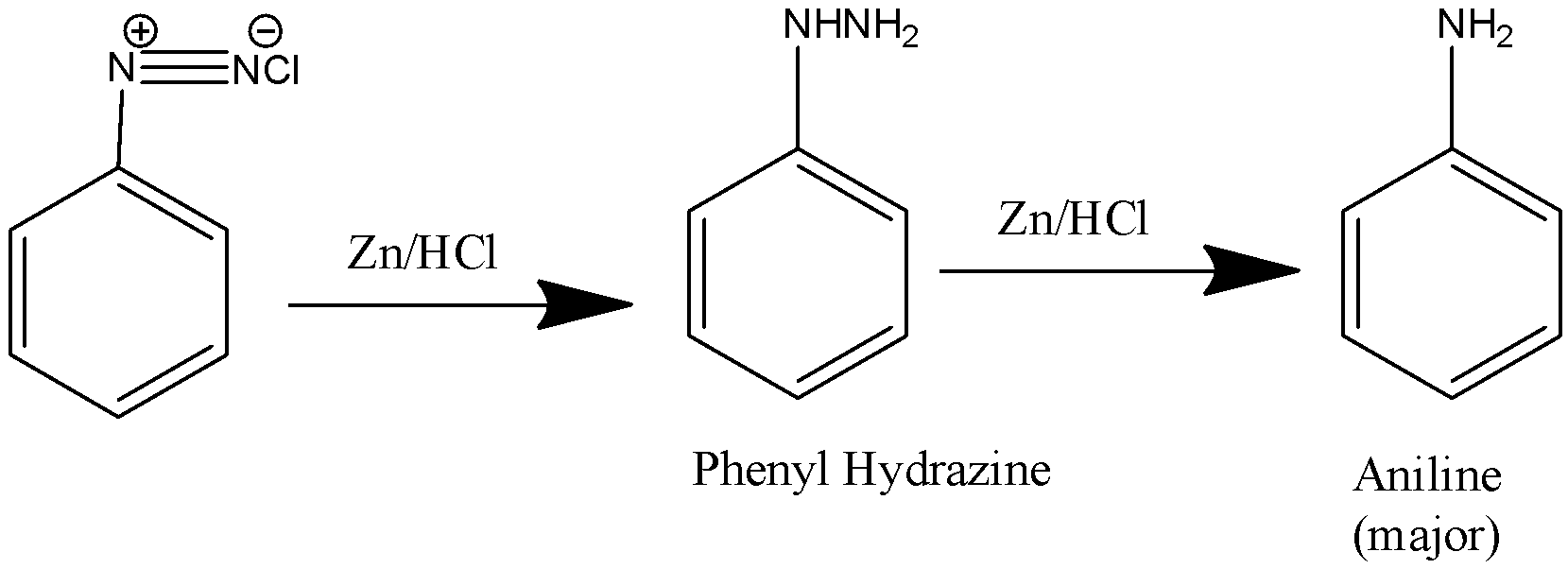
Note: Zinc and hydrochloric acid is a very strong reducing agent that is why the reaction proceeded in two steps. In case the question had told that the reducing agents were taken in a very small amount, then the major product formed would have been phenyl hydrazine and answer would have been changed to B. Also, some amount of phenyl hydrazine is also formed when we take the reducing agent in excess, but in answer we always prefer to write the product which is the major one.
