Question
Question: Complete hydrolysis of starch gives: A) glucose and galactose in equimolar amounts B) galactos...
Complete hydrolysis of starch gives:
A) glucose and galactose in equimolar amounts
B) galactose and fructose in equimolar amounts
C) glucose only
D) glucose and fructose in equimolar amount
Solution
Consider the monosaccharide units present in starch. Thy hydrolysis of a polysaccharide gives monosaccharides as the final product.
Complete answer:
We can represent starch with chemical formula (C6H10O5)n . Starch is a polymer or a polysaccharide. The monosaccharide unit present in starch is α−D− glucopyranose unit.
Starch is insoluble in cold water, but We can prepare a colloidal solution of starch by grinding and soaking. On partial hydrolysis of starch and glycogen, We can obtain the disaccharide maltose and a low molecular weight dextran. Upon hydrolysis of starch We can get two fractions. The first fraction is around 20% of the hydrolyzed product. This fraction contains amylose that is water-soluble.
Amylose contains a linear chain that is made from several thousand glucose units. The second fraction that We obtain is called amylopectin. It contains around one million glucose units. Amylopectin is a branched polymer and is water-insoluble. Hence, when we subject starch to complete hydrolysis, We get α−D−glucose as the sole product.
Let us write the structure of α−D− glucopyranose as shown below:
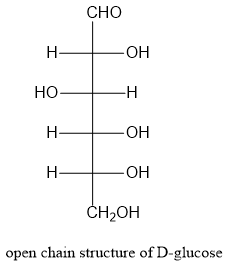
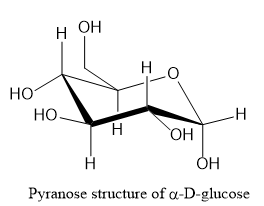
Note: Starch is a high molecular weight polysaccharide consisting of amylose and amylopectin. Amylose is a linear polymer and is around 20% of starch. Amylopectin is branched polymer and is around 80% of starch.
