Question
Question: Compare the structures of \[S{O_3}\] to \[P{F_3}\] and explain why they have different molecular sha...
Compare the structures of SO3 to PF3 and explain why they have different molecular shapes?
Solution
We need to know that the atomic number of oxygen is 8. The atomic number of fluorine is 9. The mass number of oxygen is 16. The mass number of fluorine is 18.99. The symbol of fluorine is F. Fluorine is one of the halogens in the periodic table. The symbol of oxygen is O. The atomic number of sulphur is 16. The atomic number of phosphorus is 15. The mass number of sulphur is 32. The mass number of phosphorus is 30. The symbol of sulphur is S. The symbol of phosphorus is P.
Complete answer:
The molecular formula of sulphur trioxide is SO3.
The molecular formula of phosphorus trifluoride is PF3.
The structure of sulphur trioxide is given below,
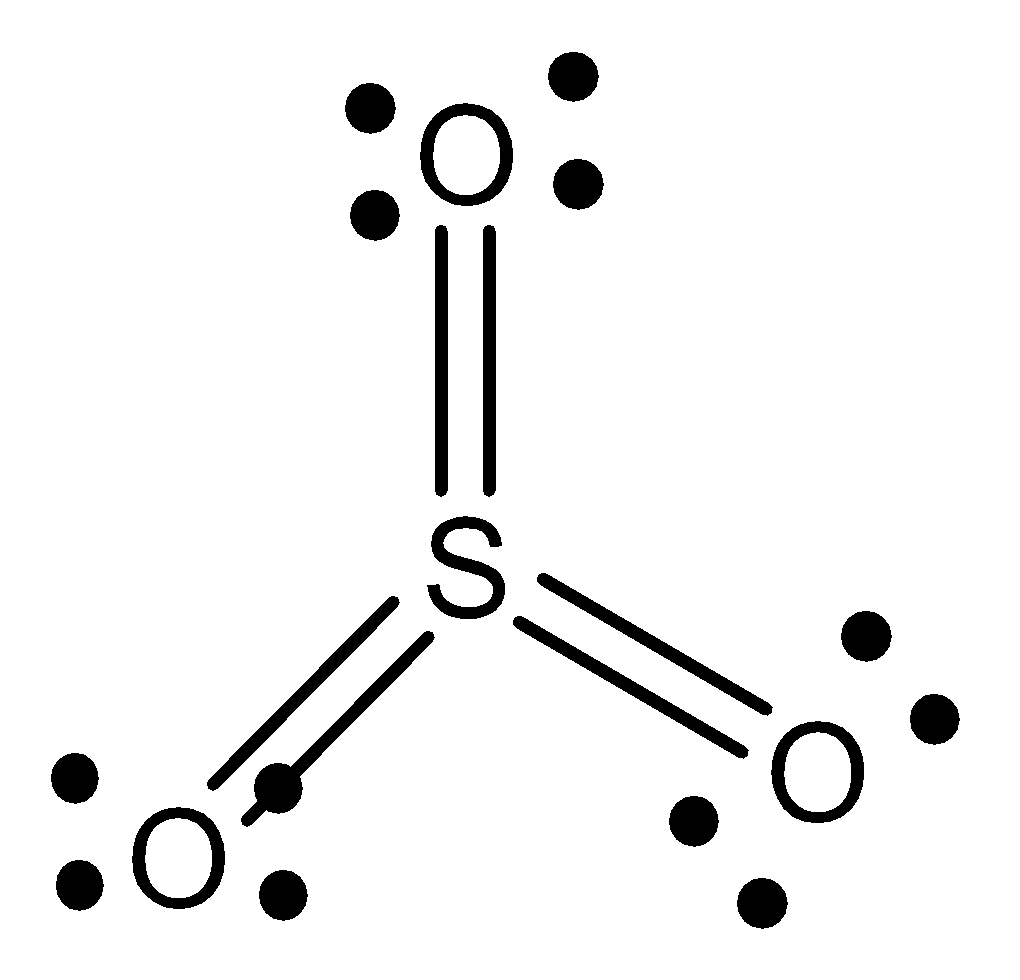
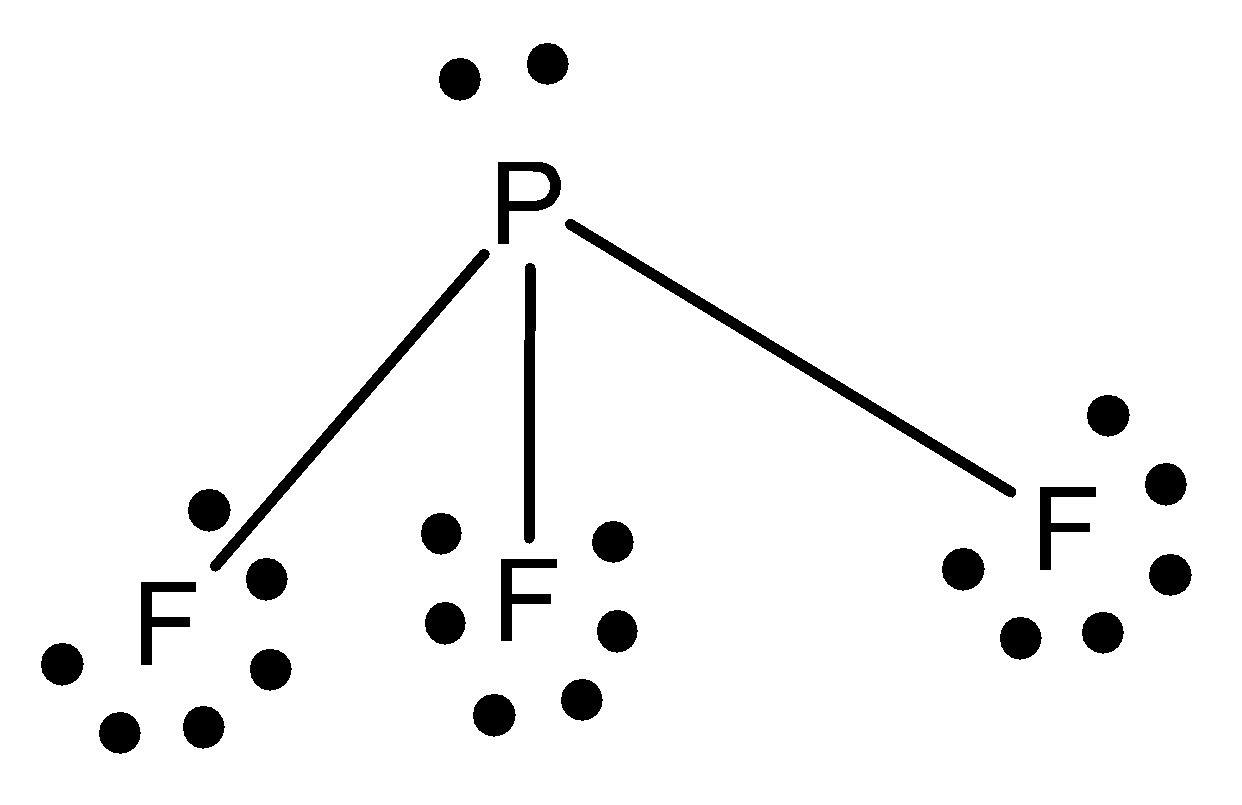
The structure of sulphur phosphorus trifluoride is given below,
The hybridisation of sulphur trioxide is sp2.
The hybridisation of phosphorus trifluoride is sp3.
The geometry of sulphur trioxide is trigonal planar.
The geometry of phosphorus trifluoride is tetrahedral.
The shape of sulphur trioxide is trigonal planar.
The shape of phosphorus trifluoride is pyramidal.
Sulphur trioxide and phosphorus trifluoride have different molecular shapes, because of the geometry and hybridisation of the molecules.
Sulphur trioxide is non-polar, because of the trigonal planar shapes all the polarity of the molecules comes to zero due to all the dipole moment of the molecule is cancel each other and phosphorus trifluoride is polar, because of the pyramidal shape all the polarity of the molecules not comes to zero due to all the dipole moment of the molecule is not cancel each other.
Note:
As we know that in chemistry, periodic tables play a vital role. In the periodic table there are totally 118 elements. In the periodic table there are totally 18 columns and 7 rows. The columns are called groups. Hence, 18 groups in the periodic table. The rows are called periods. Hence, totally 7 period in the table. The atomic number of the element is nothing but the number of electrons or number of protons. The mass number of the atom is nothing but the sum of the number of protons and number of neutrons.
