Question
Question: Compare the structures of \[{{H}_{2}}O\] and \[{{H}_{2}}{{O}_{2}}\] ....
Compare the structures of H2O and H2O2 .
Solution
Hint : In both the structures there are only single bonds present. The bond angle has changed due to the presence of lone pairs. Due to their bonding, they can form a complicated structure.
Complete step by step solution :
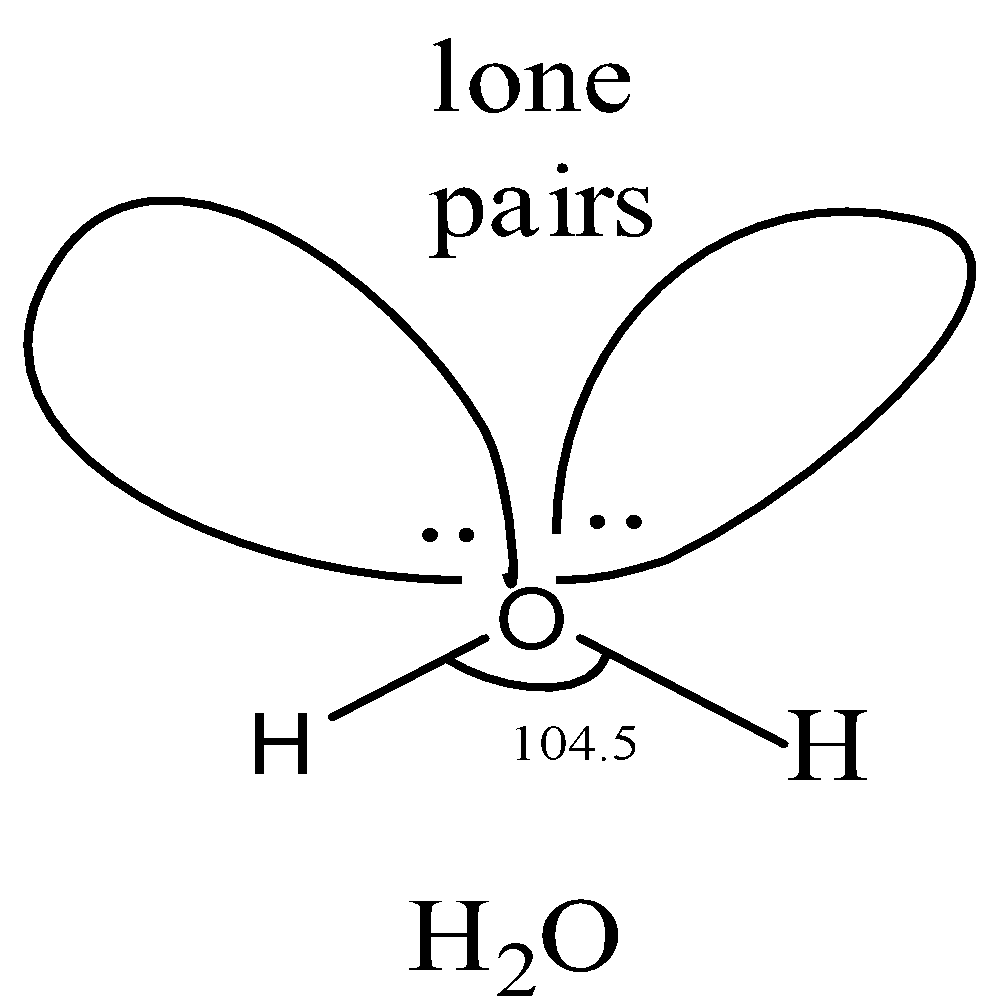
Let us study the structure of H2O.
In H2Omolecule, the oxygen atom is and hence has four sp3−hybridized orbitals. Two of thesesp3−orbitals are half-filled and hence overlap with hydrogen to form two while the other two contain a lone pair of electrons each. Since the oxygen atom is sp3−hybridized, therefore, the geometry of the molecule should be tetrahedral and the bond angle should be 109.5∘. But experimentally, the oxygen atom is surrounded by two shared pairs, and two lone pairs of the electron. But according to VSEPR theory, lone pair-lone pair repulsion is greater than bond pair-bond pair repulsion. As a result, the angle decreases to104.5∘.
The bond length is 95.7pm.
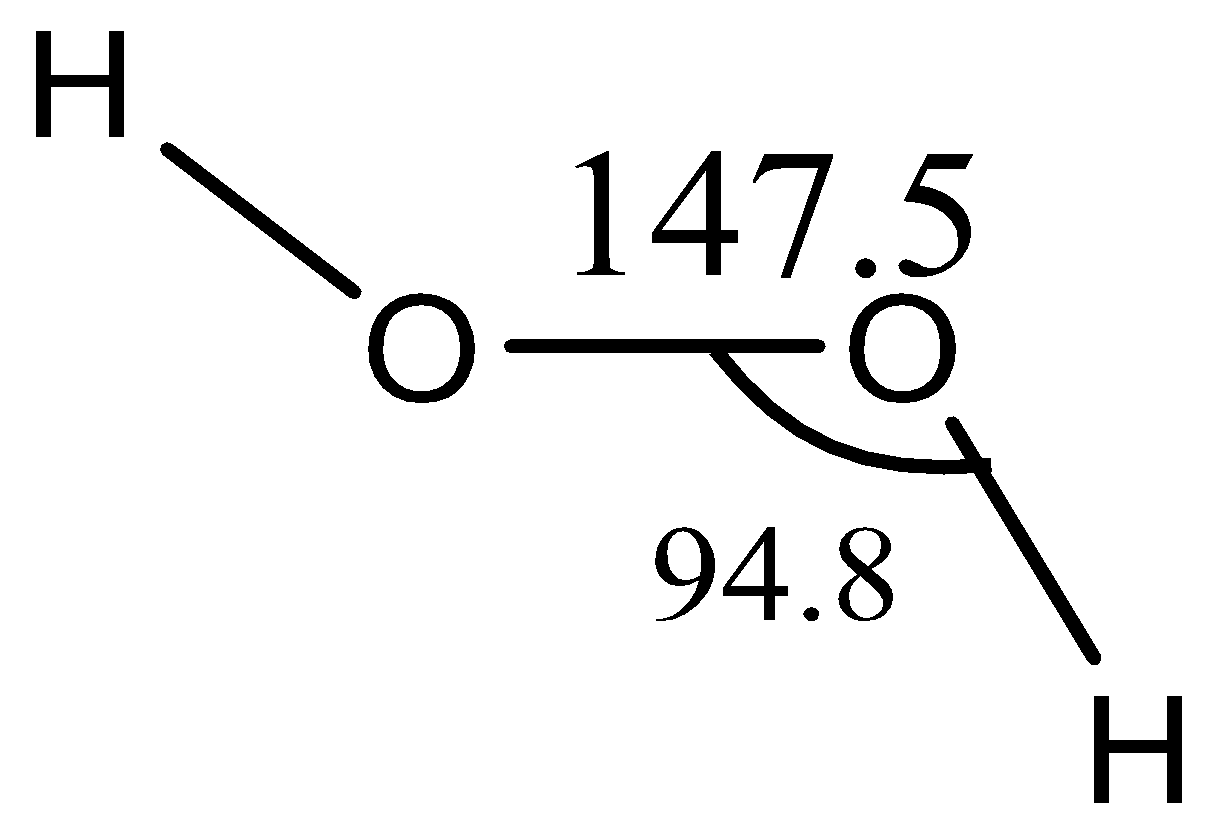
Now, the structure of H2O2
Hydrogen peroxide is a nonpolar molecule. The two oxygen atoms are linked to each other by a single covalent bond and each oxygen is further linked to a hydrogen atom by a single bond. The two bonds are, however, in different planes due to repulsion between different bonding and antibonding orbitals. The dihedral angle between the two planes being 111.5∘and in the gas phase reduces to 90.2∘. The bond length is 147.5pm and 95pm.
Note : Don’t get confused that if the hybridization is then the shape would be tetrahedral, lone pairs repulsion is a factor that should be considered. Water has a planar structure whereas hydrogen peroxide has a non-planar structure.
