Question
Question: Compare the structures of Diamond and Graphite....
Compare the structures of Diamond and Graphite.
Solution
The difference in structures of Diamond and graphite also changes physical as well as chemical properties. Both diamond and graphite are allotropes of carbon. Allotropes are compounds which are chemically the same but vary in their physical properties.
Complete step by step answer:
Before differentiating the properties and structures of diamond and graphite, let us look at carbon. It belongs to theII period and XIV group of the periodic table. Therefore, it is a metalloid which means its electronegativity and electropositivity are well balanced. Being the first member of its group, carbon is the smallest size and does not have d-orbitals. All of these properties make it an element which has the highest self-linking property. It also forms strong bonds with metals, nonmetals and metalloids. Diamond and graphite are large molecules of carbon which do not have a specific formula. Their size completely depends on the extent of their physical structures.
Let us look at the differences between diamond and graphite via a table:
| DIAMOND | GRAPHITE |
|---|---|
1. The hybridisation of carbon here is sp3. Therefore it has a tetrahedral structure.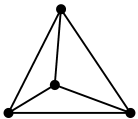 2. All four valencies of carbon are filled. 3. It has a crystal structure and therefore is very hard.4. Behaves as an insulator because there are no free electrons present for the conduction of electricity.5. The molecular arrangement of the carbon molecules is three dimensional in nature because of the tetrahedral geometry. 2. All four valencies of carbon are filled. 3. It has a crystal structure and therefore is very hard.4. Behaves as an insulator because there are no free electrons present for the conduction of electricity.5. The molecular arrangement of the carbon molecules is three dimensional in nature because of the tetrahedral geometry. | 1. The hybridisation of carbon is sp2. The structure is trigonal planar.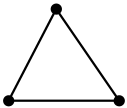 2. Only three valencies of carbon are filled.3. It is soft in nature and can easily fall apart.4. Can conduct electricity because it has one free electron per sp2 hybridised carbon.5. The molecular arrangement is two dimensional forming a plane of carbon atoms with trigonal planar geometry. These planes slip over one another without any strong bonding between them. 2. Only three valencies of carbon are filled.3. It is soft in nature and can easily fall apart.4. Can conduct electricity because it has one free electron per sp2 hybridised carbon.5. The molecular arrangement is two dimensional forming a plane of carbon atoms with trigonal planar geometry. These planes slip over one another without any strong bonding between them. |
Note: There are many significant physical differences in diamond and graphite, but the chemical properties are similar because both are composed of the same element (carbon) than their macrostructure and other conditions that gave birth to their physical differences.
