Question
Question: Compare the heats of combustion above compounds and stability. 
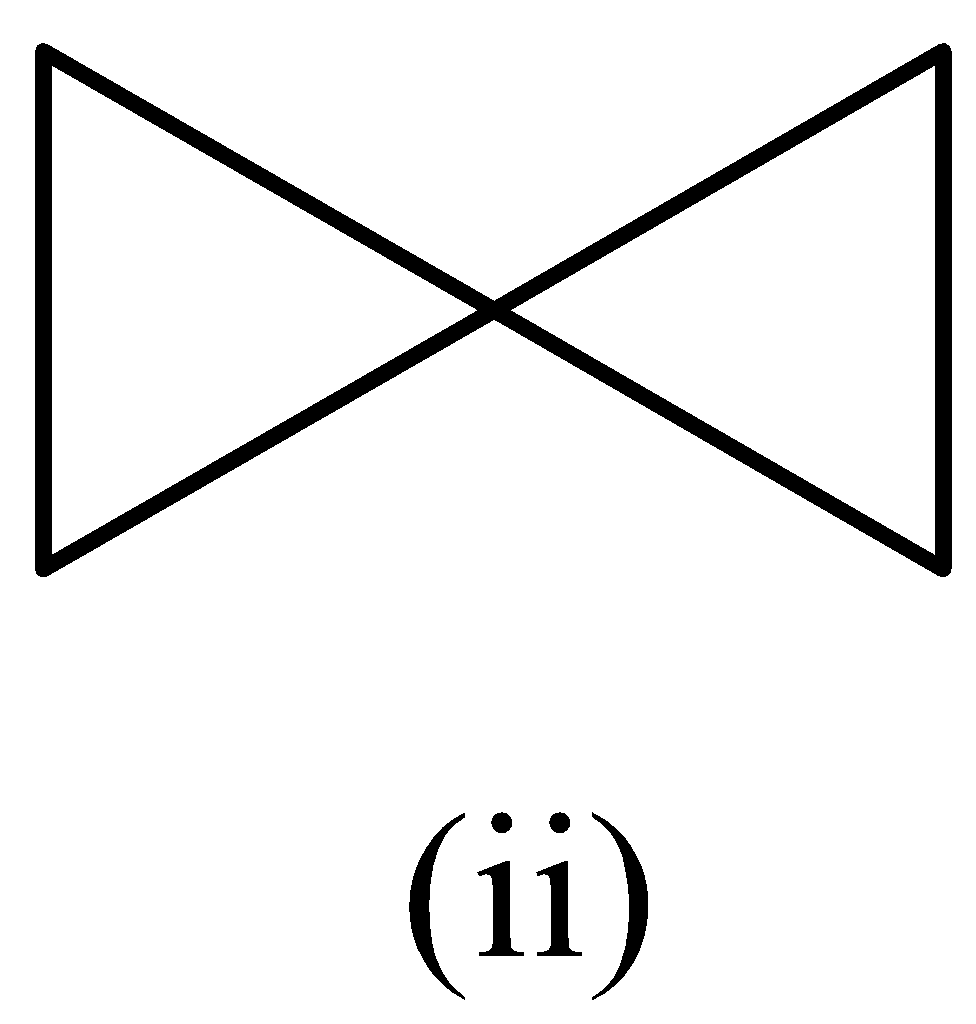
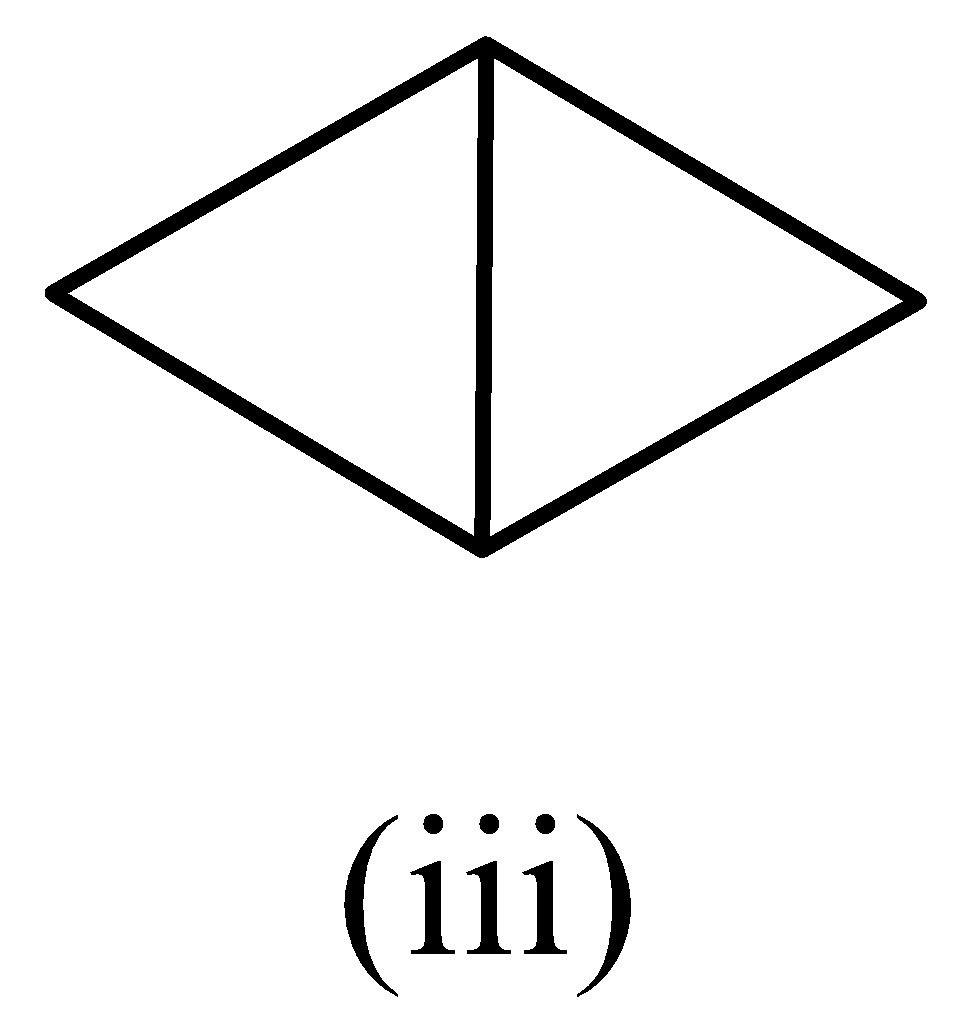
A.(i) > (ii) > (iii)
B.(i) > (iii) > (ii)
C.(ii) > (i) > (iii)
D.(ii) > (iii) > (ii)
Solution
We know that heat of combustion is also called enthalpy. In a chemical reaction, both the formation of new bonds and breaking of old bonds takes place. But when the old bonds are broken some energy is required. The reactant molecule must have some amount of energy to state the chemical reaction. In combustion reaction, the generation of heat is due to the energy necessary for breaking an old bond is lower than energy required by formation of new bonds.
Complete answer: We have to know that the heat liberated on complete combustion of one mole of a substance is known as heat of combustion and such reactions are called Exothermic.
Heat of combustion for different saturated hydrocarbons is determined with an increasing number of carbon atoms in a molecule, there would be an increase in heat of combustion. This is obviously because of more carbon existing for burning and more number of bonds undergoing changes.
The larger hydrocarbons with increasing carbon chains are tougher to ignite. This is due to the vaporization of bigger molecules aren’t easily. The combustion reaction is enabled if the oxygen and the hydrocarbon are mixed as gases. Bigger molecules contain more Van Der-Waals attractions that lend to be difficult for them to break away from their neighbors and make them into a gas.
We have to remember that the number of carbon atoms is directly proportional to the heat of combustion. More the number of atoms of carbons, the more the heat of combustion.
In structure (i), the number of carbon atoms is six.
In structure (ii), the number of carbon atoms is five.
In structure (iii), the number of carbon atoms is four.
The structure (i) would have the highest heat of combustion, followed by structure (ii), and the heat of combustion would be less for structure (iii). The order is given as,
(i) > (ii) > (iii)
Therefore, the option (A) is correct.
Note: We can also predict the heat of combustion using the primary, secondary, and tertiary hydrogens. The carbon bond with primary hydrogen would be stronger when compared to carbon bond with secondary hydrogen, and carbon bond with tertiary hydrogen. If the number of primary hydrogen is more, then the heat of combustion would be less. If the number of hydrogen is similar for all types, then we have to consider other properties such as angle strain etc.
