Question
Question: Compare the boiling points of the given compounds. 
Solution
If the molecules have intermolecular hydrogen bonding then the boiling of those compounds is high. Because of the presence of intermolecular hydrogen bond molecules won’t evaporate at lower temperature. To break this type of intermolecular hydrogen bonding there is a need for higher temperature means high boiling point.
Complete answer:
- In the given question there are three types of amine compounds.
- We have to find the order of boiling points of the given molecules.
- The given molecules are as follows.
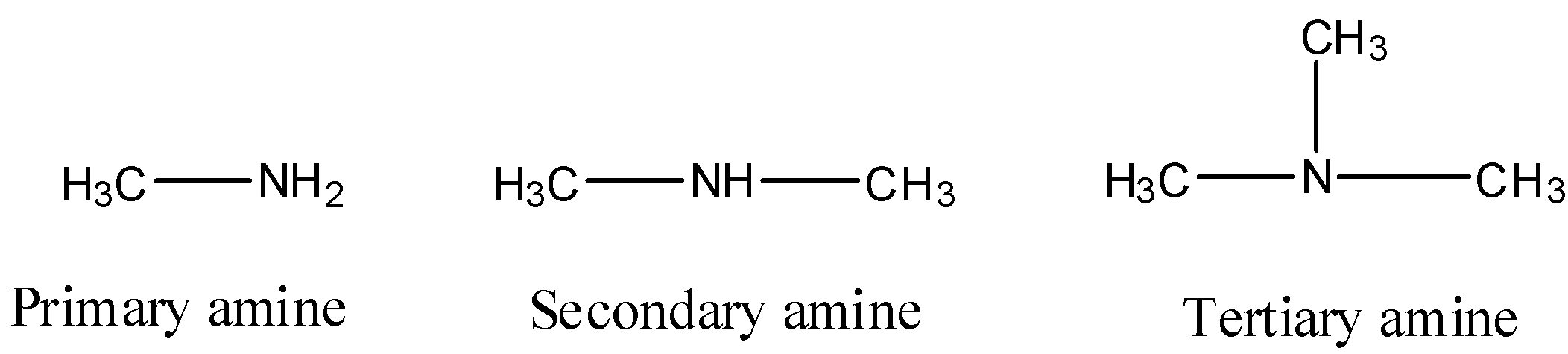
- The given compounds contain primary amine, secondary amine and tertiary amine.
- The intermolecular hydrogen bonding is high in case of primary amines because primary amines are simple and the nitrogen can form intermolecular hydrogen bonding with remaining primary amines.
- Coming to intermolecular hydrogen bonding in case of secondary amines. In case of secondary amines intermolecular hydrogen bonding is possible but it is a little bit weak compared to primary amines. Because in the case of secondary amines the number of substituents around the nitrogen is more when compared to primary amines.
- Coming to tertiary amines, the intermolecular hydrogen bonding is very less in this case. Because the nitrogen in tertiary amines is surrounded by the alkyl group and won’t allow to form intermolecular hydrogen bonds with others in the surroundings.
- So, the order of boiling point of the given compounds is as follows.
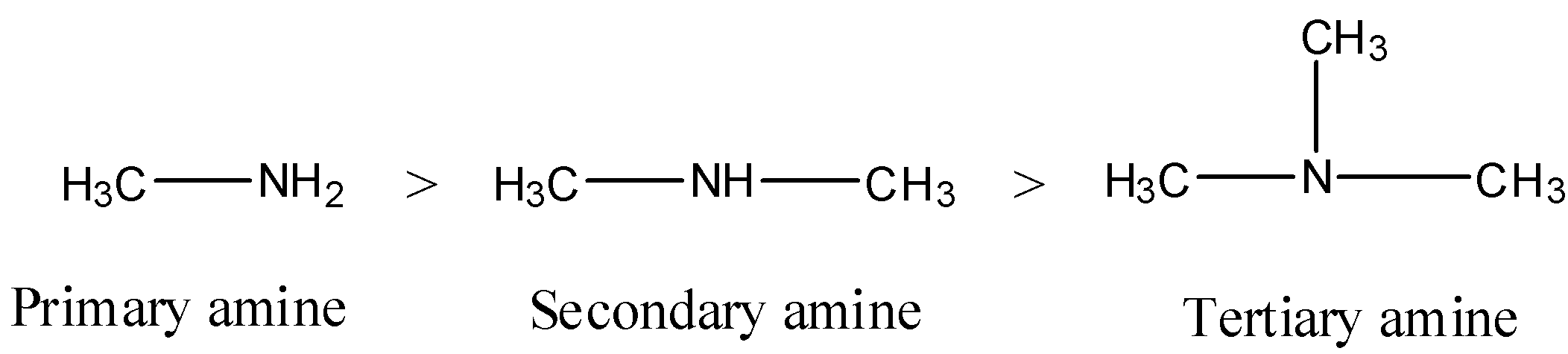
- Therefore the boiling points of the given compounds are as follows.
I > II > III.
Note:
The primary amines have a higher boiling point than secondary and secondary amines have high boiling point when compared to tertiary amines. The boiling points of the amines are going to depend on the intermolecular hydrogen bonding forming capability.
