Question
Question: Compare Stability:...
Compare Stability:
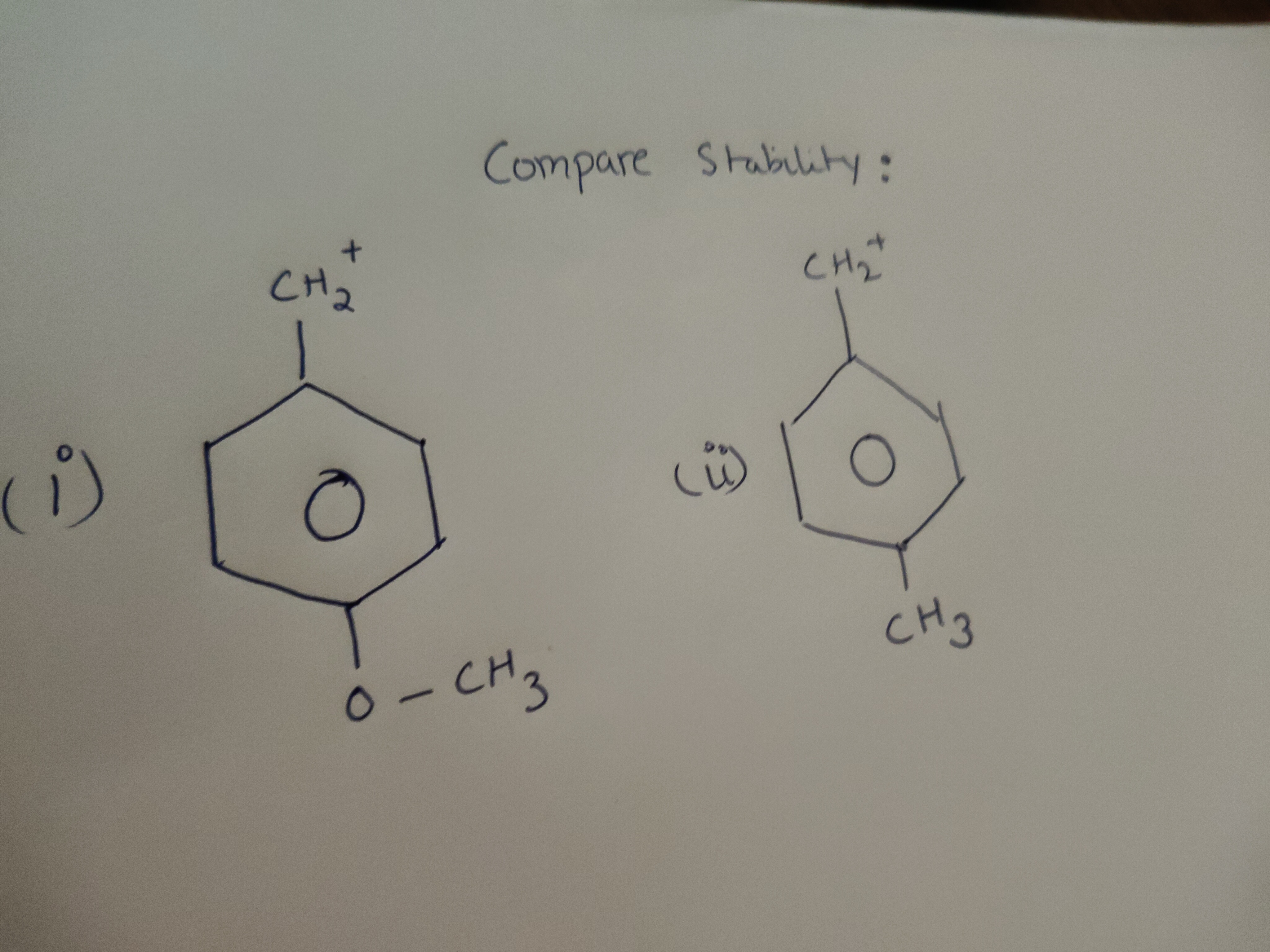
Carbocation (i) is more stable than carbocation (ii).
Carbocation (ii) is more stable than carbocation (i).
Both carbocations have similar stability.
Stability cannot be determined from the given information.
Carbocation (i) is more stable than carbocation (ii).
Solution
The stability of carbocations is enhanced by factors that delocalize or disperse the positive charge. Both species are benzyl carbocations, stabilized by resonance with the adjacent benzene ring.
-
Carbocation (i): This is a para-methoxybenzyl carbocation. The methoxy group (-OCH3) is a strong electron-donating group through resonance (+R effect) due to the lone pairs on the oxygen atom. This donation of electron density into the benzene ring effectively delocalizes the positive charge on the benzylic carbon. A key resonance structure places the positive charge on the oxygen atom, which is highly stabilizing despite oxygen's electronegativity.
-
Carbocation (ii): This is a para-methylbenzyl carbocation. The methyl group (-CH3) stabilizes the carbocation primarily through the inductive effect (+I effect) and hyperconjugation. Hyperconjugation involves the overlap of C-H sigma bonds with the empty p-orbital of the carbocation.
Comparison: The resonance donation from the methoxy group in carbocation (i) is a significantly more powerful stabilizing mechanism than the inductive and hyperconjugative effects of the methyl group in carbocation (ii). Therefore, carbocation (i) is more stable than carbocation (ii).
