Question
Question: Compare bond dissociation energies of \[{H_a}\], \[{H_b}\], \[{H_c}\] and \[{H_d}\] in the given com...
Compare bond dissociation energies of Ha, Hb, Hc and Hd in the given compound.
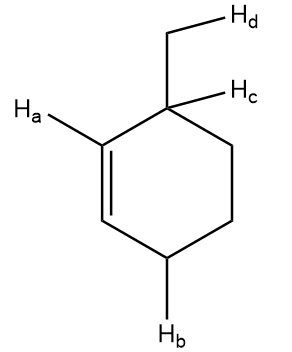
Solution
Bond dissociation energy: The amount of heat released in the homolytic cleavage of a covalent bond is termed as bond dissociation energy. In diatomic molecules, the value of bond dissociation energy is equal to the value of bond energy of the molecules. It can be used to compare the strength of chemical bonds.
Complete answer:
Free radical: When homolytic cleavage of a covalent bond takes place, electrons are distributed in such a manner that after breaking the bond, each atom has an unshared electron which is known as free radical.
Let us have a look on the free radicals formed on the homolytic cleavage of marked atoms:
Free radical formed on homolytic cleavage of C−Ha is as follows:
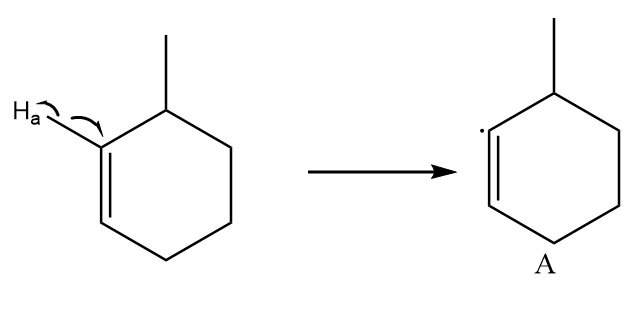
As the unshared electron is present on an electronegative atom i.e., sp2 hybridized atom. So, it is the least stable free radical.
Free radical formed on homolytic cleavage of C−Hb is as follows:
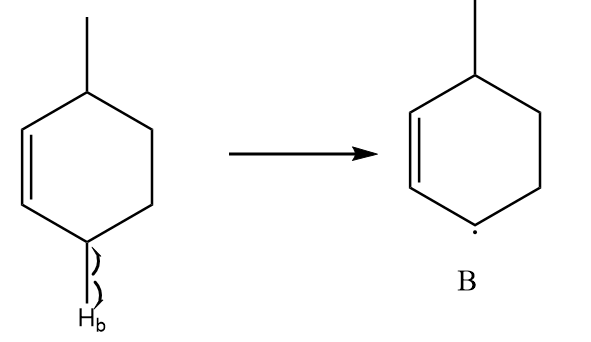
The free radical formed in structure B participates in resonance and there are a total three alpha hydrogens present in the structure. So, it is a relatively stable free radical than A.
Free radical formed on homolytic cleavage of C−Hc is as follows:
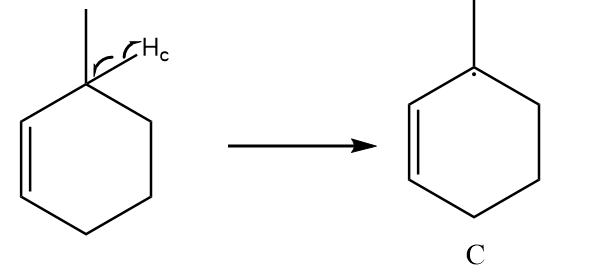
The free radical formed in structure C participates in resonance and there are a total five alpha hydrogens present in the structure. So, it is the most stable free radical.
Free radical formed on homolytic cleavage of C−Hd is as follows:
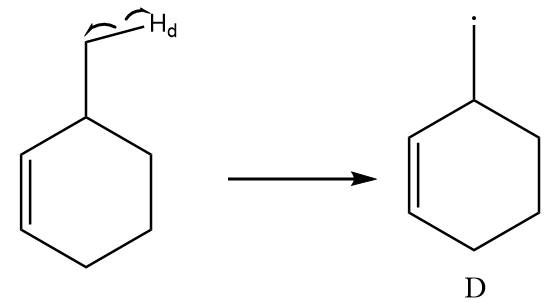
The free radical formed in structure D does not participate in resonance and there is only one alpha hydrogen present in the structure. So, it is a relatively less stable free radical than B and C.
Therefore, the order of stability of free radical formed is as follows:
C>B>D>A
As bond dissociation energy is inversely proportional to stability. Hence, the correct order for bond dissociation energy for given hydrogen atoms is as follows:
Hc<Hb<Hd<Ha
Note:
It is important to note that the more the stability of free radicals, the more easily the bond can be broken and therefore lesser will be the energy required to dissociate the bond. Hence, greater the stability of free radical, lesser will be its bond dissociation energy.
