Question
Question: Compare all the proposed models of an atom....
Compare all the proposed models of an atom.
Solution
As we know that, atoms consist of fundamental particles as electron, proton and neutron. The arrangement of these fundamental particles in an atom follows according to the models given by the various scientists.
Step by step answer: An atom is very tiny and consists of an electron, proton and neutron. The arrangement of these fundamental particles in an atom follows according to the models given by the various scientists as electrons revolve around the nucleus in circular motion, protons and neutrons are situated in the nucleus.
To know about the above arrangements, there are various models proposed by scientists. Let’s discuss them.
Thomson’s model of an atom-
After the discovery of electrons and protons, the scientists started thinking of arranging these particles of an atom. The first model was proposed by J.J Thomson known as Thomson’s atomic model.
J.J Thomson proposed that an atom consists of a uniform sphere in which positive charge is uniformly distributed. the electrons are embedded into it in such a way as to give the most stable electrostatic arrangement. This model is also known as the raisin pudding model or plum pudding model or watermelon.
Rutherford model of an atom-
In order to understand the arrangement of electrons and protons in an atom, Rutherford and his students performed a series of experiments known as Rutherford’s scattering experiments.
According to this experiment-
Most of the alpha particles passed through the gold foil undeflected. It means that there must be very large empty space within the atom.
A small fraction of alpha particles get deflected through small angles. It means that there is a heavy positively charged mass present in an atom. Moreover, this mass must be occupying a very small space within the atom because only a few alpha-particles suffered large deflections.
Very few did not pass through the foil at all the but suffered large deflections or even came back suffering a deflection of 180o. It means that there is the direct collision with the heavy positively charged mass. This heavy positively charged mass that occupies a very small volume is called a nucleus as we can see in the diagram below.
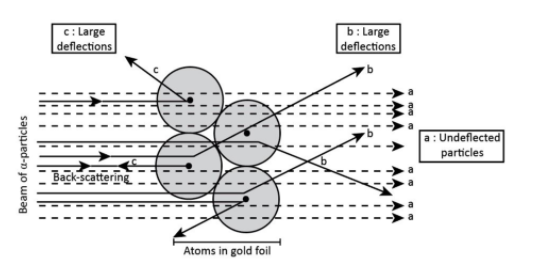
Note: The radius of the atom is found in the order to 10−10m and the radius of the nucleus is found in the order to 10−15m. It must be noted that most of the space in an atom is empty and the total mass is concentrated at a central point called as Nucleus.
