Question
Question: Compare acidic strength of the following : 
Solution
Acid strength depends on the ease to lose the proton. Electron releasing groups decrease the acidic strength whereas electron withdrawing groups increase the acidic strength. Moreover, the meta- substituted phenols are least acidic and para-substituted phenols are the most acidic in nature and after para comes the ortho-substituted phenols. Now you can easily identify the order of acidic strength.
Complete step by step answer:
The above given all the compounds below to the phenol family. So first let’s discuss what phenols are? Phenols are the hydroxyl derivatives of the hydrocarbons in which the hydroxyl group -OH is directly attached to the carbon atom of the aromatic ring.
-Phenols are colourless, crystalline solids or liquids and have characteristic phenolic odour and are sparingly soluble in water but completely soluble in alcohols, ethers etc. and their boiling point is also very high.
-And now coming to the acid strength. By the acidic strength, we mean the ease to release the hydrogen ion. In -OH bond in phenols, Oxygen atom is highly electronegative in nature and pulls the electron pair shared between them ( i.e. between the oxygen and the hydrogen atom ) towards itself and results into the release of the hydrogen ion i.e. proton and is thus, acidic in nature. Electron releasing groups such as methyl group, ethyl group etc. decrease the acidic strength and the electron withdrawing group such as nitro group, halogens etc. increases the acidic strength. The more the number of electron withdrawing groups attached to the phenol, more is the acidic acid and vice-versa. Now, considering to the statement;
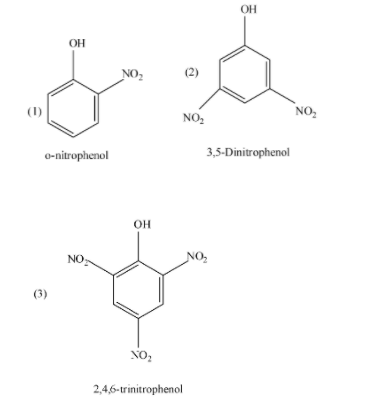
(1) In o-nitrophenol, there is intramolecular H-bonding (i.e. within the molecule) and the release of hydrogen ion is very difficult and hence, it has less acidic character but more than the meta-substituted phenols.
(2) In 3,5-dinitrophenol, there are two electron withdrawing groups which are present at the meta position and at the metal position, there are no such conjugating structures as present in ortho and para positions and hence, 3,5-dinitrophenol is less acidic than the o-nitrophenol.
(3) In 2,4,6-dinitrophenol, there are three electron withdrawing groups, that withdraws the electrons from the benzene and benzene ring then withdraws electron from the oxygen atom, so, the electron density decreases on the oxygen and pulls the electron pair towards itself and releases the proton ion. Hence, 2,4,6-dinitrophenol is the most acidic from all the given compounds.
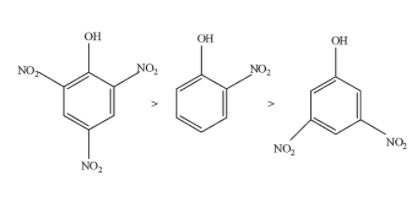
Hence, the decreasing order of acidic strength is as
Note: Phenols are more acidic then the alcohols due to the dispersal of the negative charge and thus, turn blue litmus red and react with alkali metals and alkalis to form their salts. But phenols are weaker acid than the carboxylic acids and therefore, they do not react with the sodium carbonate and sodium bicarbonate.
