Question
Question: Column chromatography involves separation of a mixture over a column of adsorbent (stationary phase)...
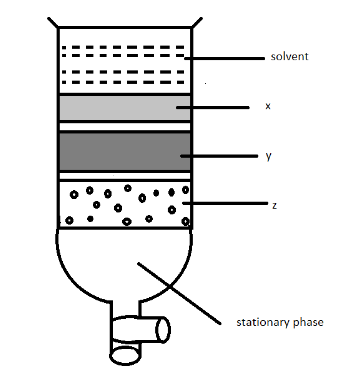
Column chromatography involves separation of a mixture over a column of adsorbent (stationary phase) packed in a glass tube. Depending upon the degree of absorption complete separation takes place. In the given column, three coloured bands x, y, z are formed. Identify the correct statement.
(A) X, y and z are absorbed to the same extent.
(B) The most readily adsorbed component is retained near the top(x).
(C) The most readily absorbed component comes down (z).
(D) x, y, z layers are formed according to the wavelengths of the colours not on the basis of adsorption.
Solution
Hint : This is the most famous instrumental method to separate the constituent compounds. While the addition of solvent from the above column, the interaction having the most intenses will be absorbed first. After this those which have the most binding power will be considered next.
Complete Step By Step Answer:
In the given diagram, there is a column which is filled by silica gel, this has a good absorption property where we know that the absorption is the intake of the matter present in a liquid by the other materials . In the given figure the (x) will get absorbed by the material giving the dark grey colour. After this (y) will be absorbed by the same material that will give us a light grey colour. After this in the last, there is a least interaction with the (z) which is having the adsorbent and adsorbate itself.
So option B is the correct answer. That is , the most readily adsorbed component is retained near the top(x).
Note :
While the addition of solvent from above column, the interaction having the most intenses will be absorbed first. After this those which have the most binding power will be considered next. This is the most famous instrumental method to separate the constituent compounds.
