Question
Question: \(CO\) is acidic in nature when it reacts with \(NaOH\) . State true or false. (A) True (B) Fals...
CO is acidic in nature when it reacts with NaOH . State true or false.
(A) True
(B) False
(C) Neither true nor false
(D) None of the above
Solution
We know that oxides are those compounds in which one or more oxygen atoms combines with another element. Carbon has two oxides that are carbon-di-oxide and carbon monoxide. Carbon monoxide is a gas that is colorless, odorless, and tasteless. Carbon mono-oxide and sodium hydroxide will react to form sodium formate.
Complete step-by-step answer: We know that oxides are those compounds in which one or more oxygen atoms combines with another element. Carbon has two oxides that are carbon-di-oxide and carbon monoxide. Carbon-monoxide has one carbon atom and one oxygen atom. They have a triple bond between them. Carbon monoxide is a gas that is colorless, odorless, and tasteless. When exposed to a concentration above 35ppm, it can be toxic to humans and animals. Carbon mono-oxide combines with hemoglobin to form carboxy-hemoglobin. Because of the formation of carboxy-hemoglobin, hemoglobin is unable to deliver oxygen to the cells in the body. Carbon monoxide has the following structure
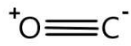
Carbon monoxide is a neutral oxide because it shows neither basic nor acidic properties. Therefore when it reacts with a strong base like NaOH it requires a high temperature and pressure. Carbon mono-oxide and sodium hydroxide will not react at standard temperature and pressure. For reaction to occur. The temperature should be between 1200C−2000C and pressure should be between 6−10 bar. The following reaction will occur:
NaOH(s)+CO 200oCHCOONa
The product formed is sodium formate. It is also known as formic acid or sodium salt. Sodium formate is used in fabric dyeing and printing processes. The salt that is sodium formate is basic and contains a weak base.
Therefore the correct answer is B.
Note: It is important to note that in carbon mono-oxide structure carbon has a slight negative charge and oxygen has a slight positive charge. This is because the total number of valence electrons in this compound is 10. Carbon shares two electrons whereas oxygen shares four electrons. Let’s draw the lewis structure of carbon mono-oxide
Formal Charge on C atom = V.E−N−21B.E.
V.E.= Number of Valence electrons
N= Number of non-bonded electrons
B.E.= Number of bonded electrons
\eqalign{
&\Rightarrow 4 - 2 - \dfrac{6}{2} \cr
&\Rightarrow 4 - 2 - 3 \cr}
Formal Charge on C atom =−1
To neutralize it, carbon monoxide undergoes resonance.
