Question
Question: \(CO_{3}^{2-}\) anions has which of the following characteristics? This question has multiple corr...
CO32− anions has which of the following characteristics?
This question has multiple correct options
A. bonds of unequal length
B. sp2 hybridization of C atom
C. resonance stabilization
D. same bond angles
Solution
As we know that CO32− is called carbonate ion. It is the simplest oxocarbon anion, that has one carbon atom which is surrounded by three oxygen atoms. It is found that CO32− is a polyatomic ion.
Complete answer:
- Let’s discuss about bond lengths of CO32− ions:
It is found that there are three identical bonds present in CO32−. We can represent the Lewis structure of CO32− as:
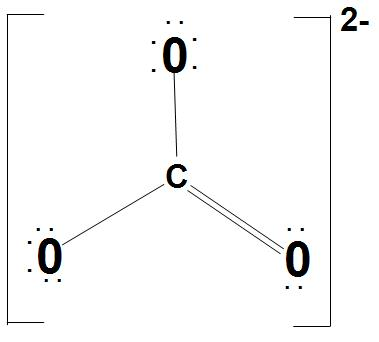
- Let’s discuss about sp2 hybridization of C atom:
It is found that the carbonate ion, carbon is bonded with one oxygen atom by a double bond and with two oxygen by a single bond. It has trigonal planar geometry which clearly mentions that the carbon is sp2 hybridized.
- Let’s discuss resonance stabilization: It is found that the carbonate anion shows resonance stabilization. We can see the resonance structures of carbonate ion with resonance hybrid:
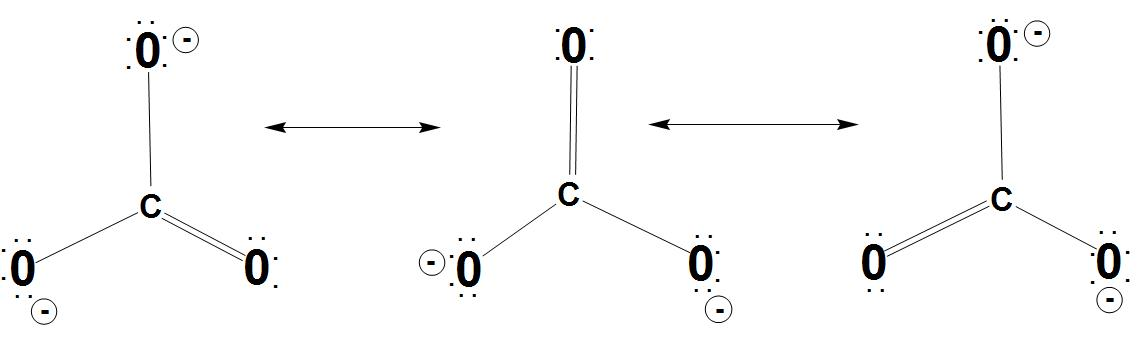
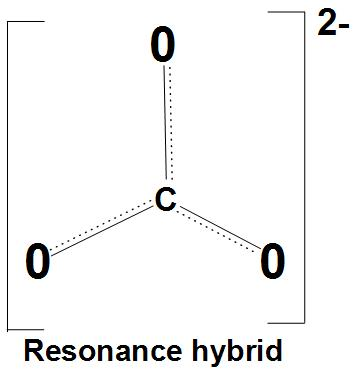
- Let’s discuss about same bond angles:
As we have discussed , the carbonate ion has trigonal planar geometry, just like BF3 , with a bond angle of 120 degree. It is found that carbonate ions have the same bond angles.
- Hence, we can conclude that the correct options are (b), (c), (d) that is CO32− has sp2 hybridization of C atom, resonance stabilization and same bond angles.
Note: - CO32− should not be confused with CO3. The main difference between both of these is that CO32− is carbonate ion, which is a stable oxocarbon anion. Whereas, CO3 is carbon trioxide, which is an unstable oxide of carbon (oxocarbon) .
