Question
Question: Classify the isomers of alcohol in \({{C}_{5}}{{H}_{12}}O\) as primary, secondary, and tertiary alco...
Classify the isomers of alcohol in C5H12O as primary, secondary, and tertiary alcohols.
Solution
Primary alcohols are those compounds in which the carbon that contains the alcohol group is attached to further one carbon atom. Secondary alcohols are those compounds in which the carbon that contains the alcohol group is attached to further two carbon atoms. Tertiary alcohols are those compounds in which the carbon that contains the alcohol group is attached to further three carbon atoms.
Complete step by step answer:
So the compound has formula C5H12O, this has five carbon atoms. This compound has 8 isomers in which we can classify them as primary, secondary, and tertiary alcohols.
Pentan-1-ol, this is a straight-chain compound, and the carbon that contains the alcohol group is attached to further one carbon atom so it is primary. The structure is given below:
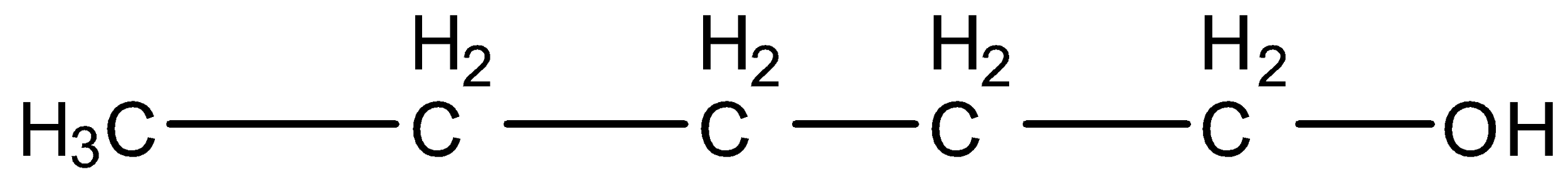
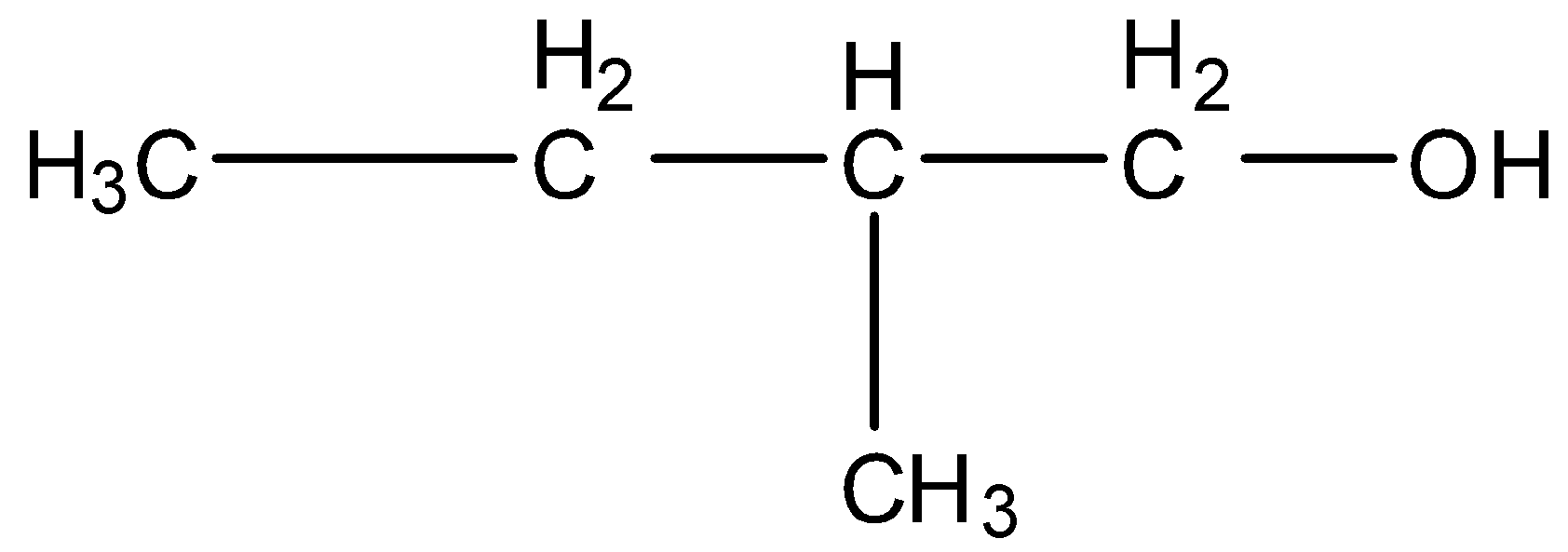
2-Methyl butanol, this is a branched form of C5H12O and the carbon that contains the alcohol group is attached to further one carbon atom so it is primary. The structure is given below:
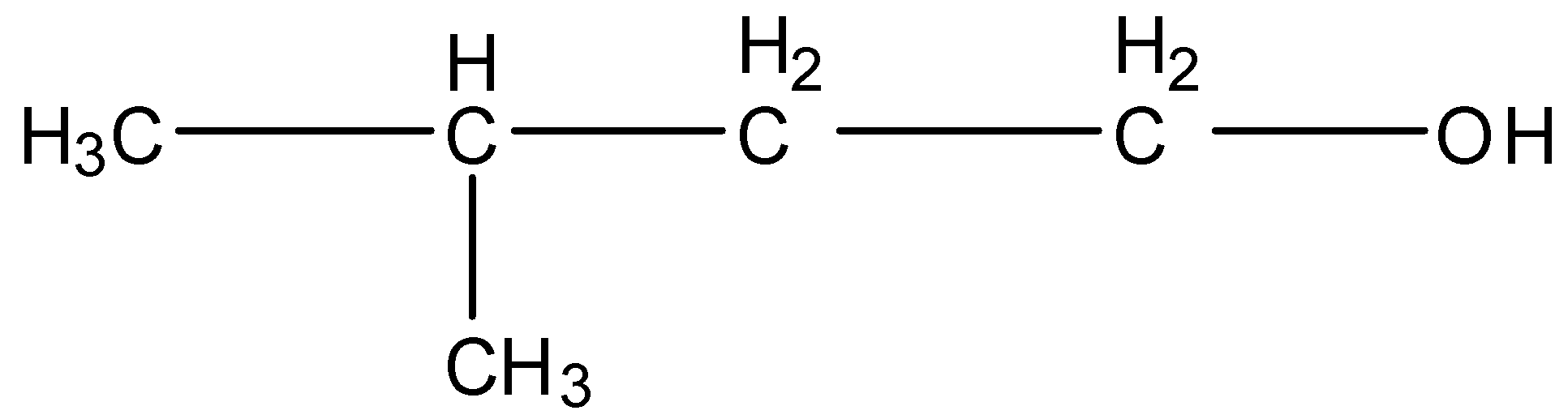
3-Methyl butanol, this is a branched form of C5H12O and the carbon that contains the alcohol group is attached to further one carbon atom so it is primary. The structure is given below:
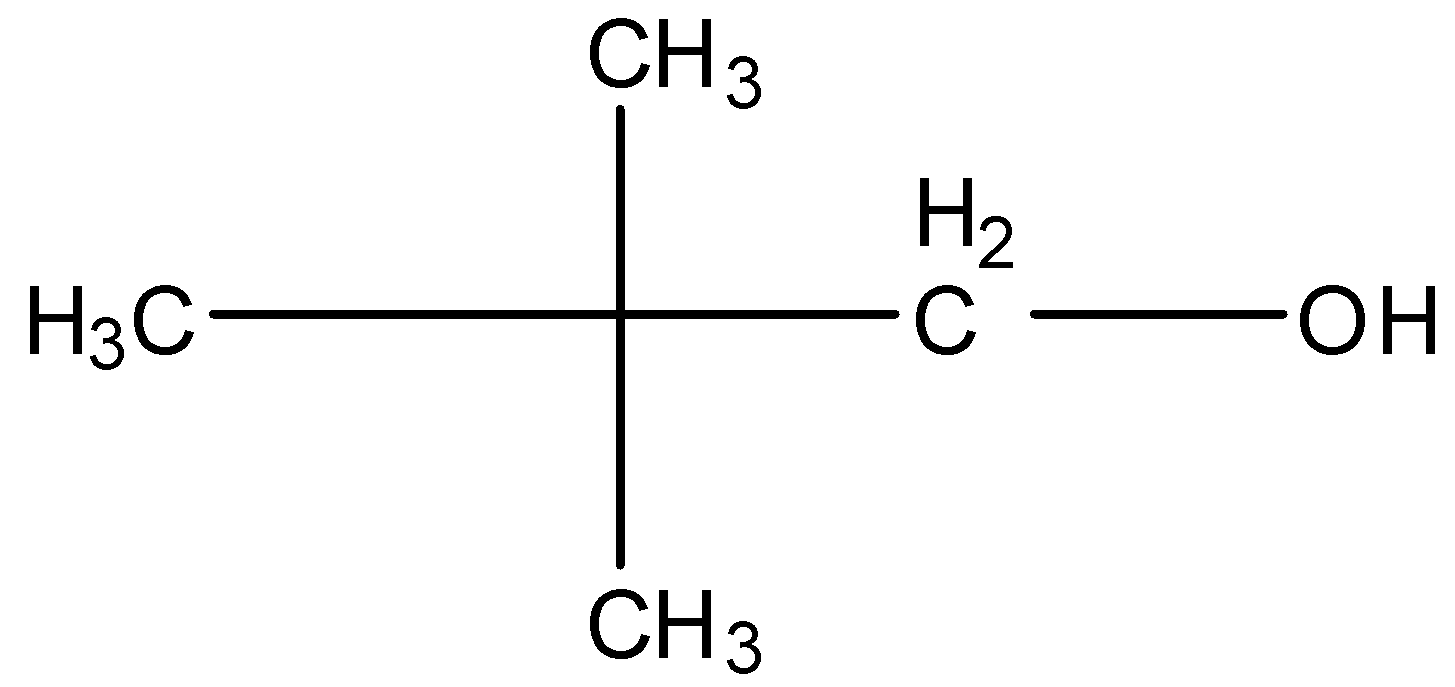
2,2-Dimethyl propanol, this is a branched form of C5H12O and the carbon that contains the alcohol group is attached to further one carbon atom so it is primary. The structure is given below:
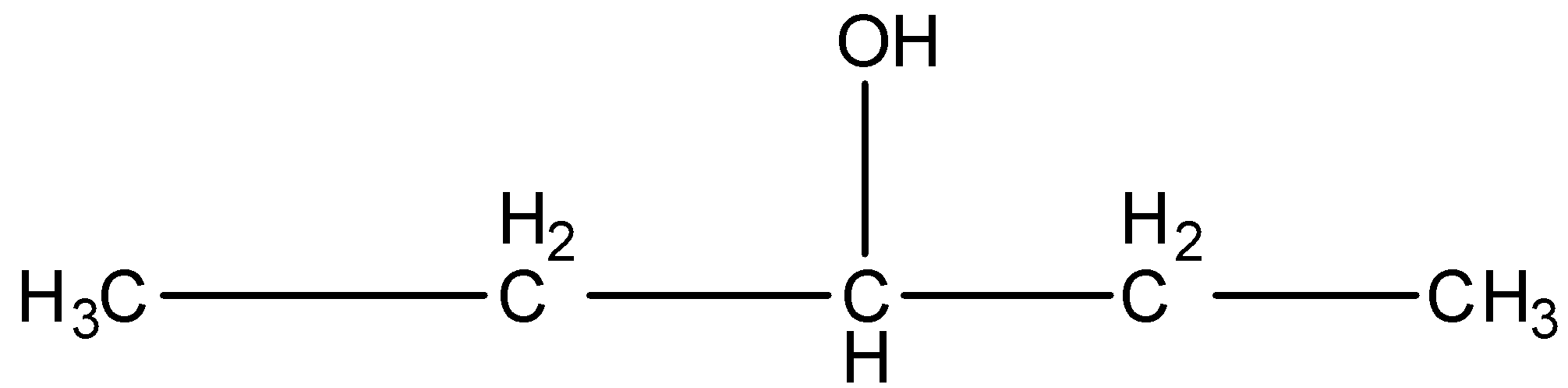
Pentan-2-ol, this is a straight-chain with alcohol at the second position, and the carbon that contains the alcohol group is attached to further two carbon atoms so it is secondary. The structure is given below:
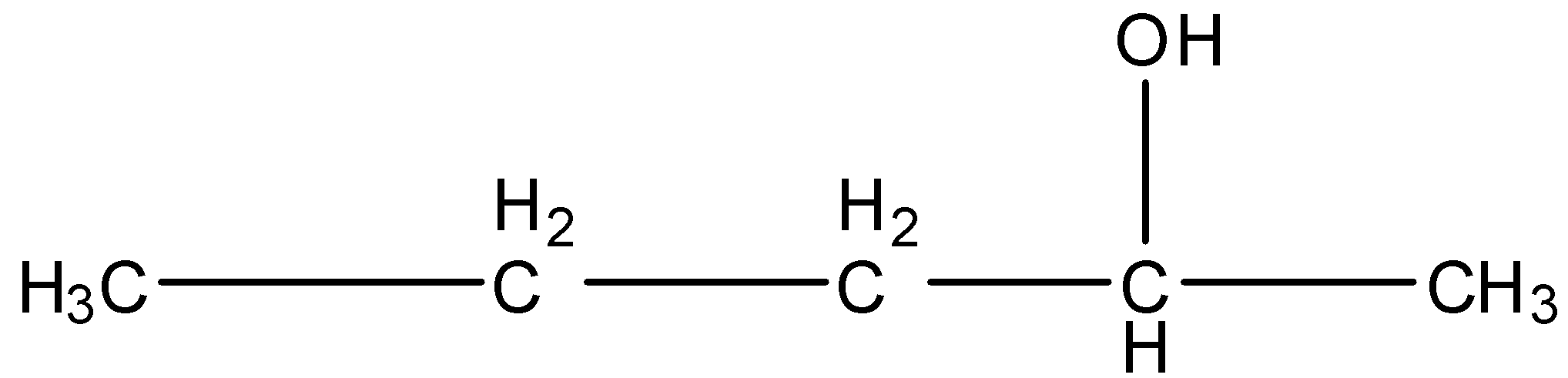
3-Methyl-2-butanol, this is a branched form, and the carbon that contains the alcohol group is attached to further two carbon atoms so it is secondary. The structure is given below:
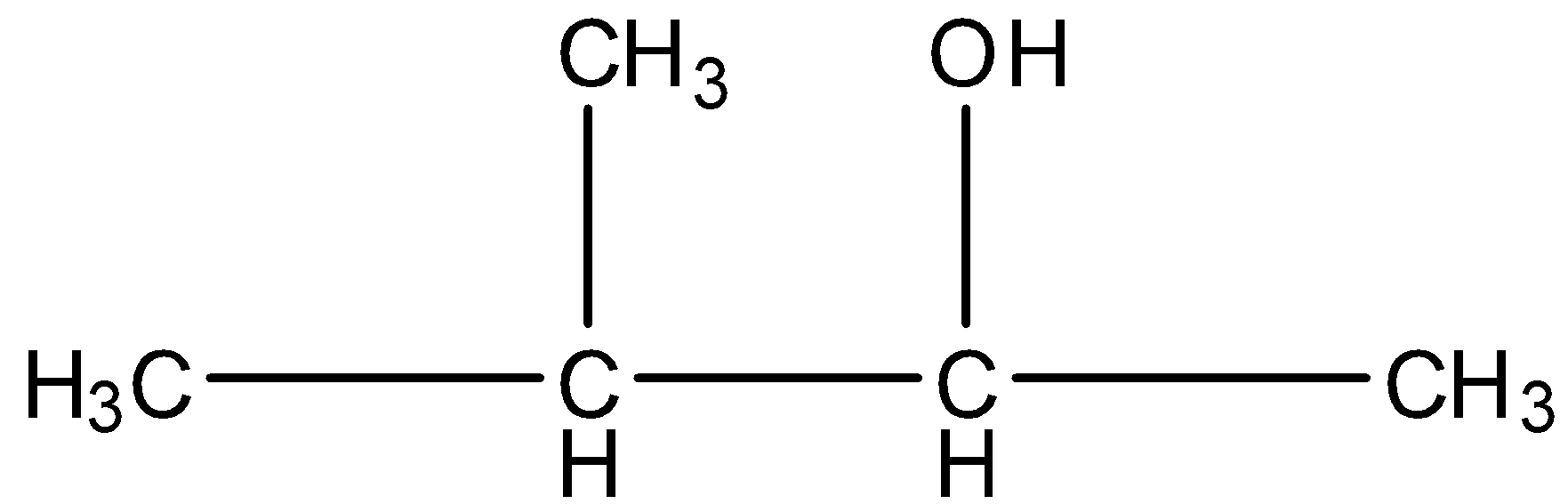
Pentan-3-ol, this is a straight-chain with alcohol at the third position, and the carbon that contains the alcohol group is attached to further two carbon atoms so it is secondary. The structure is given below:
2-Methyl-2-butanol, this is a branched form, and the carbon that contains the alcohol group is attached to further three carbon atoms so it is tertiary. The structure is given below:
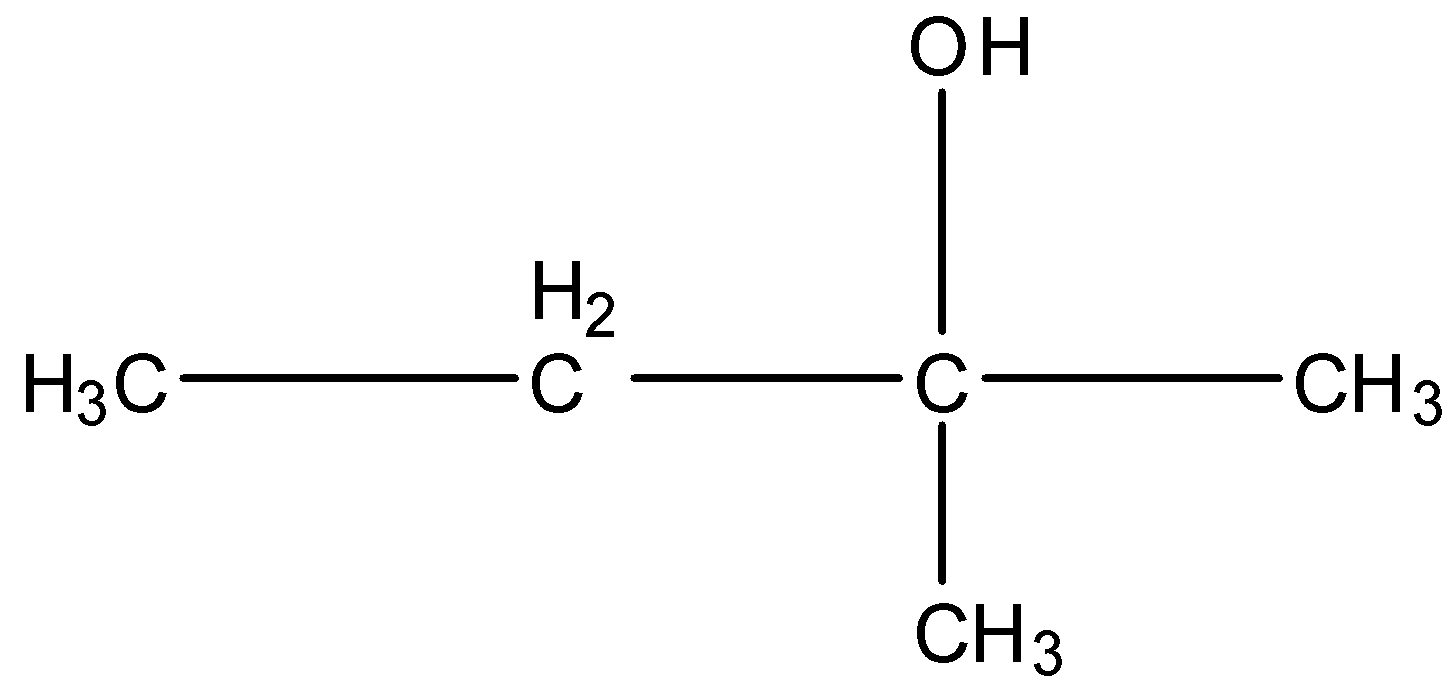
Note: You may think that the alcohol attached to the number of carbon atoms will classify them as primary, secondary, and tertiary, but it is the number of carbon atoms further attached to the carbon atom having an alcohol group.
