Question
Question: \[Cl - P - Cl\] bond angles in \(PC{l_5}\) molecule is: A. \({120^ \circ }\) and \({90^ \circ }\) ...
Cl−P−Cl bond angles in PCl5 molecule is:
A. 120∘ and 90∘
B. 60∘ and 90∘
C. 60∘ and 120∘
D. 120∘ and 30∘
Solution
Bond angles contribute the shape of a molecule. Bond angles are the angles between adjacent lines that represent bonds. The bond angle can help differentiate between linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral. It depends on the number of lone electron pairs. It is determined by the lone pair electrons repelling the bonding pairs until the ligands. Bond angles are used to little trigonometry to calculate how far apart the ligands themselves are.
Complete step by step:
According to VSEPR theory, P−Cl is bonding environments and forms two bonds in this molecule. Here we see the structure of PCl5 which is given below:
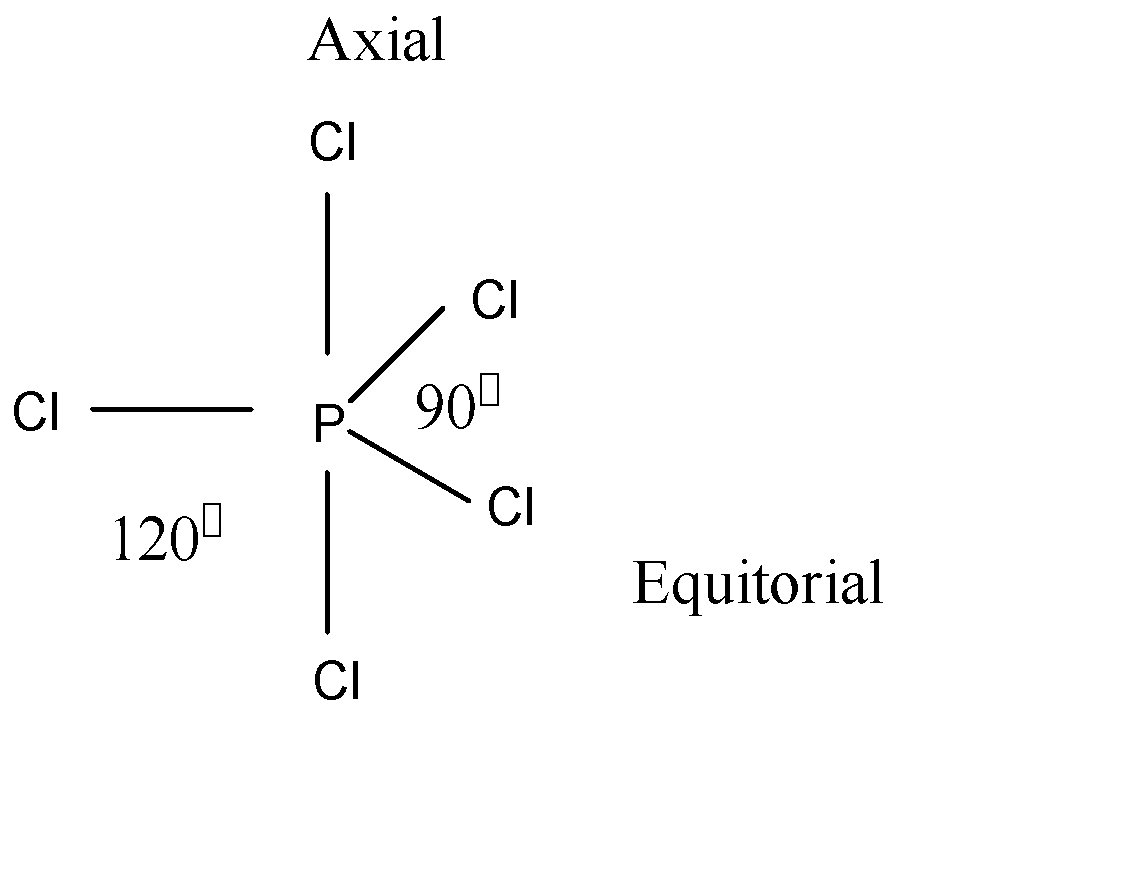
Here the central atom is P and it contributes 5 electrons. As we see the structure, there is 5 Cl to bond with P. So 5 Cl is contributing a total of 5 electrons. So, P and Cl contribute a total 10 electrons. The geometry of PCl5 is trigonal bipyramidal.
Here each two P−Cl bond makes two 90∘ and two 120∘ bond angles with the other bonds in the molecule.
Hence, option (A) is the correct answer.
Note: Here we must remember that the axial to the three equatorial bonds, so one axial bond is at right angle to 3 bonds. Therefore, 2 axial bonds will be at the right angle to 6 bonds. So, the number of right angles is 6. Each equatorial PCl5 makes two right angles. The mixing of ones, three P and one d atomic orbitals to form five sp3d hybrid orbitals of equal energy is called sp3d hybridization.
