Question
Chemistry Question on p -Block Elements
Cl?O bond order in perchlorate ion is
A
1.33
B
1.50
C
1.75
D
1.90
Answer
1.75
Explanation
Solution
Perchlorate ion (ClO4) exists in fiollowing racemic forms
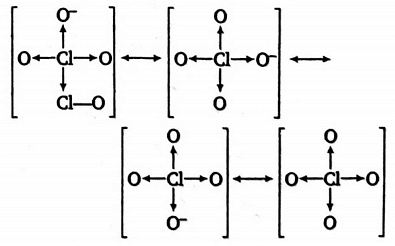
Thus, Cl?O bond order
=No. of resonating structuresTotal no. of bonds between two atoms in all resonating forms
=42×3+1=47
=1.75
