Question
Question: Chromite \[FeC{r_2}{O_4}\] is converted to \[Cr\] by following steps: \[Chromite{\text{ }}\] \[ \...
Chromite FeCr2O4 is converted to Cr by following steps:
Chromite →Na2CrO4→ Cr2O3→Cr
Reagents in Step I, II and III might be respectively:
A. airNa2CO3, Δ C,C
B. airNaOH,Δ C,Δ Al,Δ
C. airNa2CO3,ΔC,ΔC,Δ
D. conc.H2SO4,ΔNH4Cl,ΔC,Δ
Solution
When Chromite ore (FeCr2O4) is heated with sodium hydroxide (NaOH) in air, sodium chromate is obtained. Then we heat sodium chromate with carbon (C) to that chromium trioxide is obtained.
Complete answer:
Chromite ore (FeCr2O4) is heated with sodium hydroxide in air to obtain sodium chromate. It is then heated with carbon to obtain chromium trioxide. It is then heated with aluminium to form chromium. This is reduction of chromium trioxide to chromium.
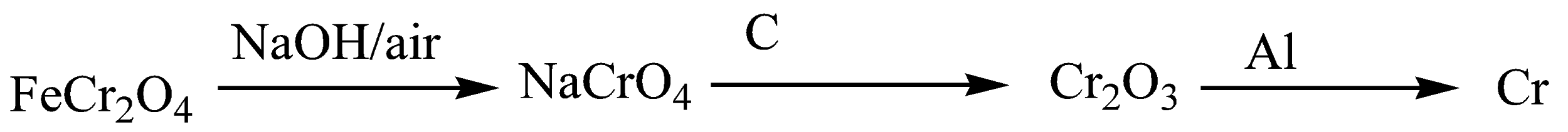
So, in the first step, sodium hydroxide (NaOH) is involved. So, I am NaOH . In the second step, it is heated with C, so II is ΔC . In the third step, it is heated with aluminium, so III is Δ Al .
So, The correct answer is option (B).
Note: Chromite is an oxide mineral composed of chromium, iron, and oxygen (FeCr2O4) . It is dark grey to black in the colour with a metallic to submetallic lustre and a high specific gravity. It occurs in basic and ultrabasic igneous rocks and in the metamorphic and sedimentary rocks that are produced when chromite-bearing rocks are altered by heat or weathering. Chromite is important because it is the only economic ore of chromium, an essential element for a wide variety of metal, chemical, and manufactured products. Many other minerals contain chromium, but none of them are found in deposits that can be economically mined to produce chromium. Chromite is sometimes slightly magnetic. This can cause it to be confused with magnetite. Chromite and ilmenite have very similar properties. Careful observations of hardness, streak, and specific gravity are required to distinguish these minerals in hand specimens.
