Question
Question: Choose the correct statement(s) (i) \[2 - \] Methylpropanoic acid & ethanol react in the presence ...
Choose the correct statement(s)
(i) 2− Methylpropanoic acid & ethanol react in the presence of conc. H2SO4 to give a compound with a fruity smell.
(ii) In[Ni(CN)4]2−, the d−orbital involved in the hybridization is dx2−y2
(iii) Solid N2O5 contains discrete units of NO2+ andNO3− .
A. I, II
B. II, III
C. I, II, III
D. I, III
Solution
The first statement belongs to the esterification reaction between an acid and an alcohol. The valence bond theory is used to determine the coordination complex structure for the second statement. The third statement belongs to the existence of N2O5 in solid phase.
Complete step by step answer:
Let us check the correctness of the given statements one by one.
(i) 2− Methylpropanoic acid & ethanol react in the presence of conc. H2SO4 to give a compound with a fruity smell.
The given reaction is an organic chemical reaction known as Fischer esterification. In this reaction, a carboxylic acid is treated with an alcohol in the presence of catalytic acid and leads to formation of an ester molecule and water. For the given substrates the 2− methylpropanoic acid or isobutyric acid is the carboxylic acid and the ethanol is the alcohol which is reacted in presence on catalytic H2SO4. The product of the reaction is ethyl isobutyrate which has a fruity smell. The reaction is
CH3CH(CH3)COOH+C2H5OHH2SO4CH3CH(CH3)COOC2H5+H2O
Hence the given statement is correct.
(ii) In [Ni(CN)4]2−, the d−orbital involved in the hybridization is dx2−y2.
In order to find the d− orbital involved, the hybridization of the given complex is to be determined. The given complex is a tetrahedral complex of nickel. Let the oxidation state of the central atom nickel is x. It is an anionic complex with two negative charges.
x+4(−1)=−2
x=+2.
Nickel is an element in the periodic table with atomic number 28 and electronic configuration [Ar]3d84s2 . The electronic configuration of Ni2+ is[Ar]3d8 . Thus the four cyanide atoms occupy the one d, one s and two p-orbitals of the Ni2+ ion.
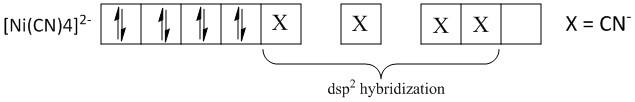
The dx2−y2 orbital is placed in the xy plane with the lobes directed along the x and y axes. Thus the dsp2 hybridization favors a planar geometry where all the four hybridized orbitals lie along the xy plane. Thus dx2−y2 orbital is used for dsp2 hybridization and the statement is correct.
(iii) Solid N2O5 contains discrete units of NO2+ and NO3−.
N2O5 is known as Dinitrogen pentoxide. The compound is a binary nitrogen oxide, which consists of nitrogen and oxygen. It exists as colourless crystals with melting point of 41∘C and boiling point of 47∘C.
N2O5 exists in the form of two structures depending on the phases of the compound. In the solid phase it is a salt called nitronium nitrate. It consists of separate nitronium cations [NO2]+ and nitrate anions [NO3]− . The compound in the gas phase exists as a covalently bound molecule.
So, the correct answer is Option C.
Note: The concept of hybridization is important for determining the structure of the complex. The choice of d− orbitals depends on the type of hybridization and the geometry of the molecule.
