Question
Question: Choose the correct sequence for the geometry of the given molecule: borazone, borazole, \({B_3}{O_6}...
Choose the correct sequence for the geometry of the given molecule: borazone, borazole, B3O6−3 ,Fe2Cl6 and trimer of FCN.
[P stands for planar, NP stands for Non planar]
A.NP,NP,P,P,P
B.P,P,NP,NP,P
C.NP,NP,P,NP,P
D.NP,P,P,NP,P
Solution
it is required to find out the electronic configuration of the central atom of these compounds. After finding the electronic configuration it is required to decide the hybridization of the compound. Mostly all sp3, sp3d, sp3d2, sp3d3 hybridized compounds are non-planar.
Complete step by step answer:
Taking each molecule one by one we shall examine whether they are planar or not.
Borazone in general is cubic boron nitride. it has the same structure as that of a diamond. If we can recall the hybridization of diamond is sp3this means that just like the diamond, the geometry of borazone is also non planar.
Borazole is also known as inorganic benzene. It has a structure similar to benzene except in place of carbon atoms we find boron atoms thus earning the name inorganic benzene. The hybridization of carbon in benzene is sp2 and therefore, comparing the structures we can say that the hybridization of borazole being the same will mean that it is indeed, planar. The molecular formula is B3N3H6.
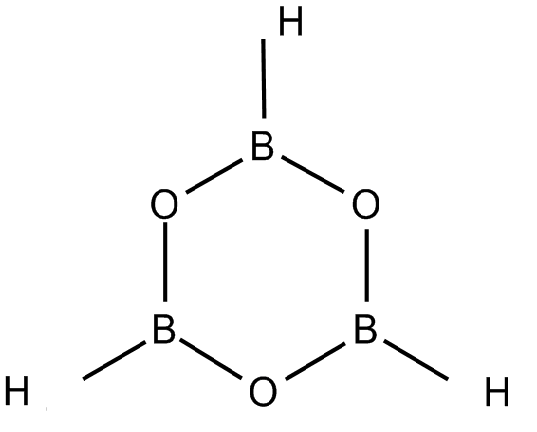
B3O6−3 is combined with hydrogen ions to form H3B3O6. This compound is known as boroxine. It is a planar molecule as the boron in this compound is joined to two oxygen atoms as shown in the diagram below.
Fe2Cl6is a dimer of ferric chloride. it is non planar. The dimer is connected to each other through a bond known as chloride bridges which means that some bonds may be out of the main plane.
Trimer of FCN which is basically three molecules of carbononitridic fluoride also known as cyanogen fluoride. It forms a ring structure during trimerization. It is similar to the structure of borazole thus making it a planar molecule.
So, the correct answer is Option D.
Note: the important point to remember is that a particular compound is said to be non planar when the surrounding atoms bonded to the central atom are not in the same plane. the surrounding atoms are usually perpendicular to the central atom. Also remember that mostly all sp3, sp3d, sp3d2, sp3d3hybridized compounds are non-planar.
