Question
Question: Choose the CORRECT option(s) regarding the given scheme ...
Choose the CORRECT option(s) regarding the given scheme
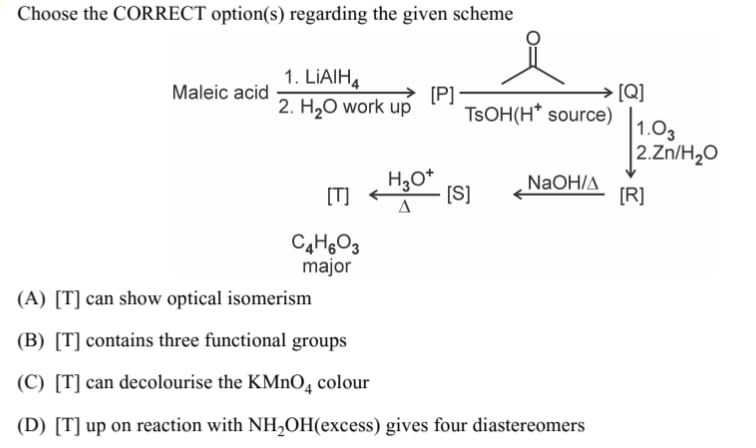
[T] can show optical isomerism
[T] contains three functional groups
[T] can decolourise the KMnO4 colour
[T] up on reaction with NH2OH(excess) gives four diastereomers
Options (A), (B) and (C) are correct.
Solution
We outline the logic as follows. The acid‐reduction of maleic acid (C₄) produces a diol which under acid catalysis cyclizes. The protonated intermediate then loses water to give an enone – a cyclic “ketone” [Q] that, according to the description, is a 2,2‐disubstituted cyclopentenone. Ozonolysis of an enone cleaves the C=C bond so that after base–acid treatment (the well–known benzilic‐acid–type rearrangement of a 1,2‐diketone) the final product [T] is obtained. Its formula C₄H₆O₃ is exactly that of an α,β–unsaturated α–hydroxy acid. (Such molecules have three distinct functional groups – a carboxyl, a hydroxyl and a C=C–conjugated carbonyl; the olefinic bond is susceptible to oxidative bleaching by KMnO₄ and the stereocentre at the hydroxyl bearing carbon impart optical activity.) Finally, since only one carbonyl group is present for conversion to the corresponding oxime, reaction with NH₂OH (even if it furnishes a pair of E/Z–isomers) cannot yield four diastereomers.
Thus the correct statements are that [T] (i) is capable of optical isomerism, (ii) contains three different functional groups and (iii) can decolourise KMnO₄.
