Question
Question: Choose the CORRECT option regarding given scheme?...
Choose the CORRECT option regarding given scheme?
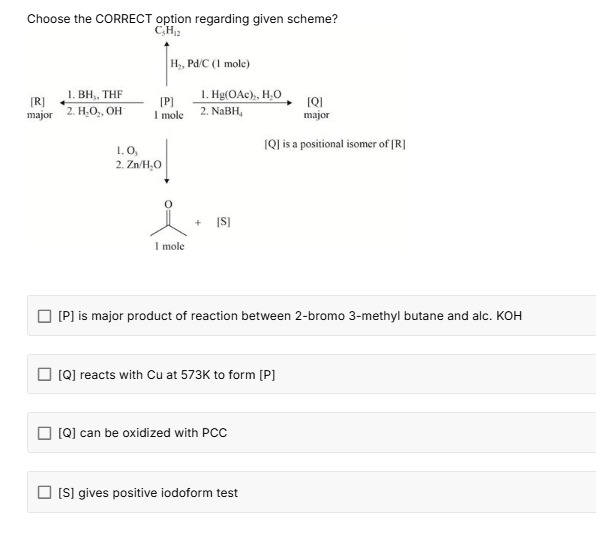
[P] is major product of reaction between 2-bromo 3-methyl butane and alc. KOH
[Q] reacts with Cu at 573K to form [P]
[Q] can be oxidized with PCC
[S] gives positive iodoform test
The CORRECT options are: [P] is major product of reaction between 2-bromo 3-methyl butane and alc. KOH, [Q] reacts with Cu at 573K to form [P], [S] gives positive iodoform test
Solution
-
Determine [P]: The reaction of 2-bromo-3-methylbutane with alcoholic KOH is an elimination reaction. The possible alkenes formed are 2-methylbut-2-ene (trisubstituted) and 3-methylbut-1-ene (monosubstituted). According to Zaitsev's rule, the major product is the more substituted alkene, which is 2-methylbut-2-ene. Thus, the first option is correct.
-
Determine [Q] and [R]: Assuming [P] is 2-methylbut-2-ene (CH3-C(CH3)=CH-CH3).
- Hydroboration-oxidation of [P] yields 2-methylbutan-1-ol ([R]).
- Oxymercuration-demercuration of [P] yields 2-methylbutan-2-ol ([Q]).
-
Evaluate Option 2: [Q] is 2-methylbutan-2-ol, a tertiary alcohol. Dehydration of tertiary alcohols with copper at 573K produces alkenes. Dehydration of 2-methylbutan-2-ol yields 2-methylbut-2-ene, which is [P]. Thus, the second option is correct.
-
Evaluate Option 3: [Q] is a tertiary alcohol. Tertiary alcohols are resistant to oxidation by mild oxidizing agents like PCC. Thus, the third option is incorrect.
-
Determine [S] and Evaluate Option 4: Ozonolysis of [P] (CH3-C(CH3)=CH-CH3) followed by reductive workup (e.g., Zn/H2O) yields acetone (CH3-CO-CH3) and acetaldehyde (CH3-CHO). From the scheme, [S] is the aldehyde product. Acetaldehyde (CH3-CHO) contains the CH3-CO- moiety and thus gives a positive iodoform test. Thus, the fourth option is correct.
