Question
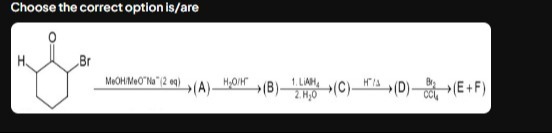
Question: Choose the correct option is/are ```latex \xrightarrow{\text{MeOH/MeO}^{-}Na^{+}(2eq)}(A)\xrightarr...
Choose the correct option is/are
\xrightarrow{\text{MeOH/MeO}^{-}Na^{+}(2eq)}(A)\xrightarrow{H_2O/H^{+}}(B)\xrightarrow{\begin{subarray}{l}1.LiAlH_4\\2.H_2O\end{subarray}}(C)\xrightarrow{H^{+}/\Delta}(D)\xrightarrow{\frac{Br_2}{CCl_4}}(E+F)
Enantiomers of trans-1,2-dibromocyclohexane
Solution
The reaction sequence starts with 2-bromocyclohexanone.
Step 1: Reaction with MeOH/MeO−Na+ (2 eq). This is a Favorskii rearrangement of an α-bromoketone. The methoxide ion abstracts an α-hydrogen from C6. The resulting enolate attacks C2, displacing Br− and forming a bicyclo[3.1.0]hexane-1-one intermediate. Nucleophilic attack of methoxide on the carbonyl carbon (C1) followed by ring opening of the cyclopropane ring (breaking the C1-C6 bond) leads to the formation of methyl cyclopentanecarboxylate. Product A: Methyl cyclopentanecarboxylate.
Step 2: Reaction with H2O/H+. Acidic hydrolysis of the ester A. Product B: Cyclopentanecarboxylic acid.
Step 3: Reaction with 1. LiAlH4, 2. H2O. Reduction of the carboxylic acid B with LiAlH4. Product C: Cyclopentylmethanol.
Step 4: Reaction with H+/Δ. Dehydration of the primary alcohol C. Protonation of the -OH group followed by elimination of water forms a primary carbocation. This carbocation undergoes a ring expansion rearrangement to form a more stable secondary carbocation in a six-membered ring (cyclohexyl carbocation). Loss of a proton from the cyclohexyl carbocation leads to the formation of cyclohexene. Product D: Cyclohexene.
Step 5: Reaction with Br2/CCl4. Electrophilic addition of bromine to the alkene D. Addition of Br2 to cyclohexene forms 1,2-dibromocyclohexane. This reaction produces a racemic mixture of trans-1,2-dibromocyclohexane. Since the question asks for E + F, it is likely referring to the enantiomers or diastereomers formed. In the addition of Br2 to cyclohexene, the addition is anti, leading to trans-1,2-dibromocyclohexane. Cyclohexene is an achiral molecule. The product 1,2-dibromocyclohexane has two chiral centers. The trans diastereomer exists as a pair of enantiomers. Therefore, E and F are the two enantiomers of trans-1,2-dibromocyclohexane.
The final products E and F are the enantiomers of trans-1,2-dibromocyclohexane.
Let's summarize the products: A: Methyl cyclopentanecarboxylate B: Cyclopentanecarboxylic acid C: Cyclopentylmethanol D: Cyclohexene E and F: Enantiomers of trans-1,2-dibromocyclohexane.
