Question
Question: Choose the correct option for - which is not the structure of \( {C_4}{H_9}Br \) (A) \( 1 - \) Br...
Choose the correct option for - which is not the structure of C4H9Br
(A) 1− Bromobutane
(B) 2− Bromobutane
(C) 1− Bromo - 2− methylpropane
(D) Isobutane
Solution
Hint : Bromobutane is a colourless liquid, but its impure sample can be seen as slightly yellow in colour. Bromobutane is an organobromine compound with a molecular formula of C4H9Br and the boiling point of around 100−205oC .
Complete Step By Step Answer:
To solve this question we should have some insight regarding the conformation of structures.
Looking at our options - 1− Bromobutane and 2− Bromobutane, let’s have a look at their structure for the better understanding –
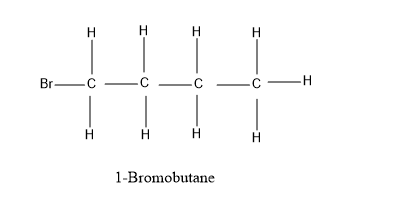
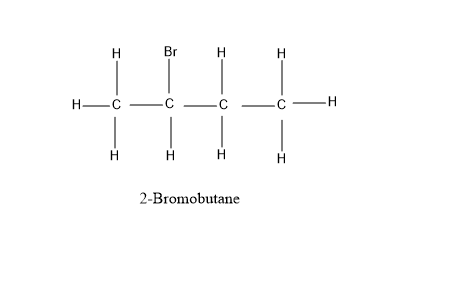
As, it is evident from the diagram above, that 1− Bromobutane and 2− Bromobutane fits completely into the formula C4H9Br . All the valencies of all the atoms are completely satisfied and have a stable structure under this formula - C4H9Br .
Similarly. 1− Bromo - 2− methyl propane – also has a stable structure satisfying all the valences of each atom.
But, coming to our option D , it is isobutane . It has a molecular formula of C4H10 . Isobutane does not consist of bromine atoms. So it does not fit in our given formula C4H9Br .
So, the correct option to our question is D i.e. isobutane.
Note :
For C4H9Br we have four possible structural isomers and they are - 1− Bromobutane and 2− Bromobutane, 1− Bromo - 2− methylpropane, these three we just read above, but the fourth isomer that is possible is – tert-butyl bromide or 2− Bromo - 2− methylpropane. These bromobutane are less soluble in water and are denser than water.
