Question
Question: Choose the correct option for the following: Assertion:\(NC{l_3}\) undergoes hydrolysis Reason:C...
Choose the correct option for the following:
Assertion:NCl3 undergoes hydrolysis
Reason:Chlorine has vacant d− orbital
(A) Both assertion and reason are correct. The reason is the correct explanation for the assertion.
(B) Both assertion and reason are correct. The reason is not the correct explanation for the assertion.
(C) The assertion is correct but the reason is incorrect.
(D) Both assertion and reason are incorrect.
Solution
We know that hydrolysis means ‘reaction with water’, so hydrolysis of nitrogen trichloride means the reaction of nitrogen trichloride with water. Nitrogen trichloride has a molecular formula NCl3 . It is a nonpolar covalent compound.
Complete answer: We know that Nitrogen trichloride has a molecular formula NCl3 . It is also called trichloramine. Nitrogen trichloride is obtained when ammonium salts like ammonium nitrate react with chlorine. We can write the structure of NCl3 as
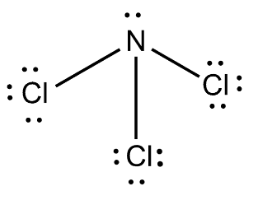
Here nitrogen and chlorine form three covalent bonds. N has an atomic number 7 and it has an electronic configuration 1s22s22p3 , we can see that p− orbital is half-filled. Chlorine has an atomic number 17 . The electronic configuration of chlorine can be written as 1s22s22p63s23p5 .
Now according to our question, the assertion is NCl3 undergoes hydrolysis. This means we have to react NCl3 with water. Hydrolysis of nitrogen trichloride is done by hot water. The following reaction will occur:
NCl3+3H2O→NH3+3HOCl
This means the assertion is correct. The products formed on hydrolysis of NCl3 are ammonia and hypochlorous acid.
From the above diagrams, we see that nitrogen needs three electrons to complete p− orbital and thus forms three covalent bonds with chlorine atoms. Now the p− orbital of nitrogen is completely filled and therefore water molecules cannot attack nitrogen to give lone pairs of electrons. Chlorine has vacant d− orbitals and thus water molecules attack chlorine. Thus the given reason is also correct.
So, the correct option is (A).
Note: We should also know that NCl3 is an unstable compound. This is because the sizes of nitrogen and chlorine are different and also the bonding between 2p of nitrogen and 3p of chlorine is not strong enough. Therefore NCl3 is unstable and explosive.
