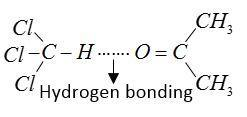Question
Question: \(CHC{l_3}\)on reaction with acetone in basic medium gives a compound used as: (A) tear gas (B) ...
CHCl3on reaction with acetone in basic medium gives a compound used as:
(A) tear gas
(B) hypnotic
(C) pesticide
(D) anesthetic
Solution
Acetone contains carbonyl group in it. Carbonyl groups undergo addition reactions. This reaction based on the concept that chloroform [CHCl3] added on carbonyl group 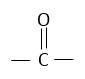 of acetone.
of acetone.
Complete step by step answer:
Let us write chemical equations for a given statement.
Chloroform [CHCl3] reacts with acetone 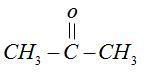 in basic medium [KOH] and undergoes an addition reactions.
in basic medium [KOH] and undergoes an addition reactions.
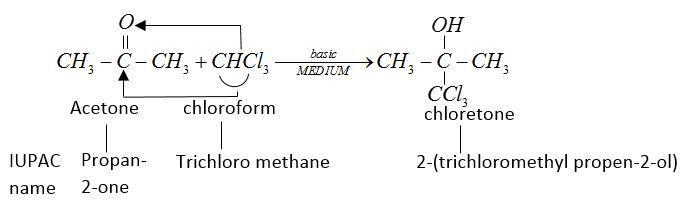
This chlorine formed is a sleep inducing drug.
Therefore, used as hypnotic.
Mechanism:
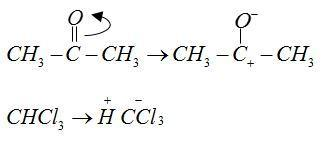
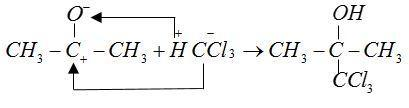
The anions of reactant CH3CO− and CCl3− form bonds with cations H+and CH3+ respectively.
Therefore, from the above explanation the correct option is (B) Hypnotic.
Additional Information:
When acetone and chloroform are mixed together heat is evolved due to formation of hydrogen bonds between chloroform and acetone. This mixture forms a non-ideal solution and shows negative deviation from Raoult’s law.
But in the presence of strong alkali it gives chlorine.
due to increased intermolecular attraction and decreases vapor pressure.
At a specific composition, acetone and chloroform will form maximum boiling azeotrope.
Azeotrope are composed of one or more liquids having very similar boiling points even having different molecules weight.
Note:
For formation of chloretone basic medium is required mixture due to hydrogen bonding present between them.