Question
Question: $CH_2 \dagger Br$...
CH2†Br
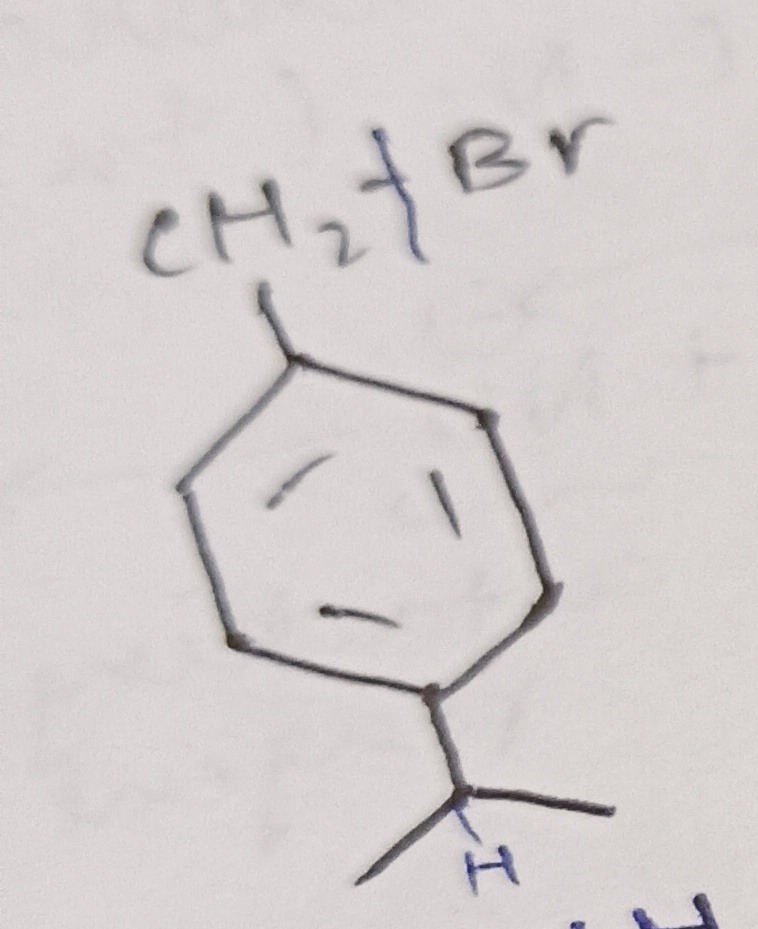
Answer
1-(bromomethyl)-4-isopropylbenzene
Explanation
Solution
The given image displays a chemical structure.
- Identify the core structure: The central part of the molecule is a six-membered ring with alternating double and single bonds, which represents a benzene ring.
- Identify the substituents:
- One substituent is clearly shown as −CH2Br. The text "CH2†Br" written above it confirms this group, with the † symbol interpreted as a bond.
- The other substituent is an isopropyl group, −CH(CH3)2, which is attached to the benzene ring by its central carbon.
- Determine the relative positions of substituents: The −CH2Br group and the isopropyl group are attached to opposite carbons of the benzene ring. This indicates a para (1,4) substitution pattern.
- Name the compound using IUPAC rules:
- The substituents are bromomethyl (−CH2Br) and isopropyl (−CH(CH3)2).
- According to alphabetical order, 'bromomethyl' comes before 'isopropyl'.
- Assign the carbon bearing the bromomethyl group as position 1.
- Since the isopropyl group is para to the bromomethyl group, it will be at position 4.
- Therefore, the IUPAC name is 1-(bromomethyl)-4-isopropylbenzene.
- Alternatively, using the common para prefix, the name can be p-(bromomethyl)isopropylbenzene.
The structure can be represented as:
The molecule is:
(Note: The mermaid diagram is a simplified representation and does not show the aromaticity within the ring explicitly as three double bonds, but rather as a cyclic structure with single bonds, which is a limitation of this specific diagram type for aromatic rings. The SMILES string is more accurate for detailed structure representation.)
The structure is a benzene ring with a bromomethyl group and an isopropyl group at para positions.
Solution: The chemical structure shown in the image is 1-(bromomethyl)-4-isopropylbenzene.
