Question
Question: CH3 HBr Peroxide $\longrightarrow$ Major product will be : ...
CH3
HBr Peroxide ⟶ Major product will be :
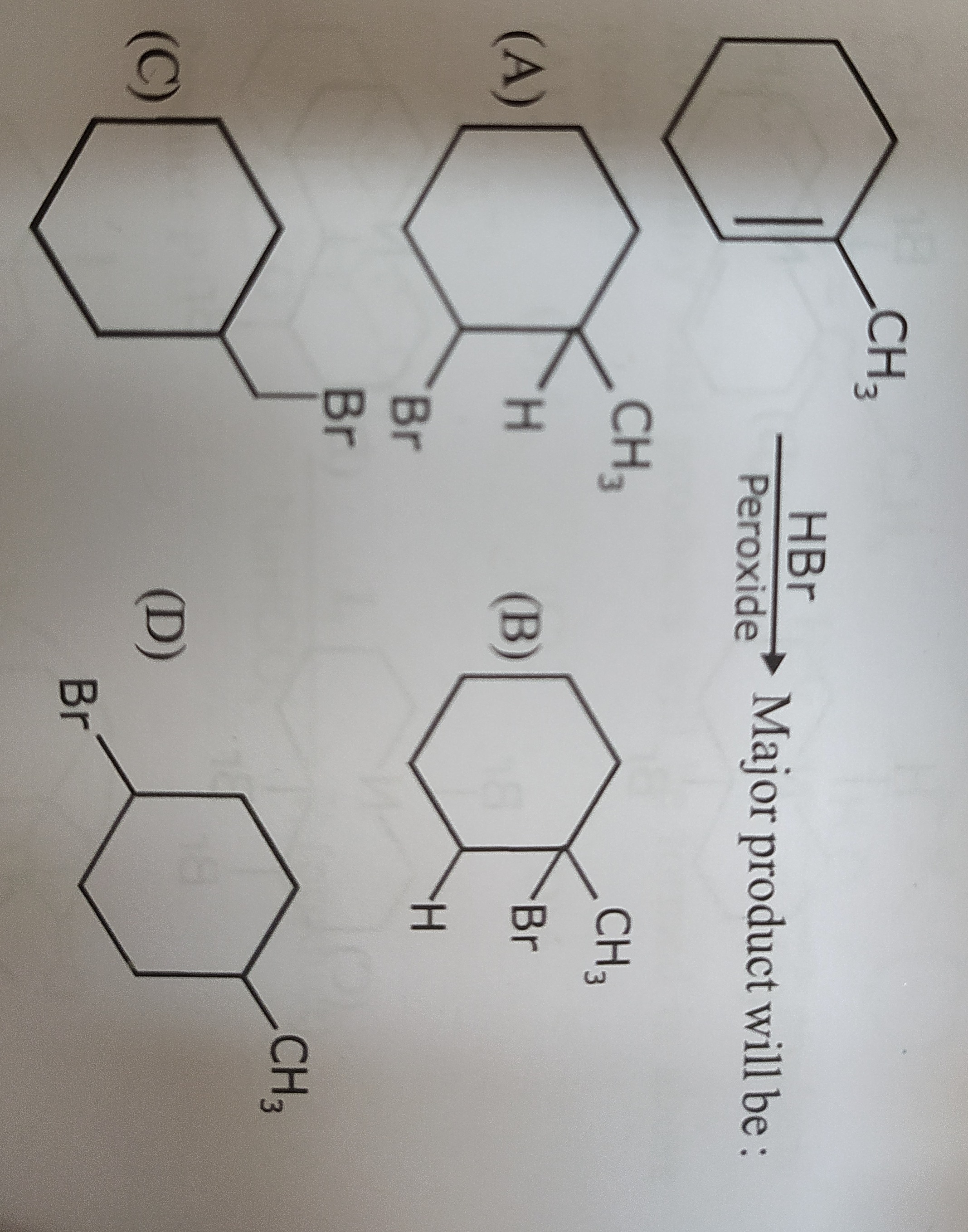
A
B
C
D
Answer
A
Explanation
Solution
The reaction of 1-methylcyclohexene with HBr in the presence of peroxide follows the anti-Markovnikov rule via a free-radical mechanism. The bromine atom adds to the less substituted carbon of the double bond (carbon with more hydrogens), and the hydrogen atom adds to the more substituted carbon (carbon with fewer hydrogens). In 1-methylcyclohexene, the carbon with the methyl group has no hydrogens, and the adjacent double-bonded carbon has one hydrogen. Therefore, Br adds to the carbon with one hydrogen, and H adds to the carbon with the methyl group. This leads to 1-methyl-2-bromocyclohexane.
