Question
Question: +NaI$\xrightarrow{Acetone}$ product; S$_{N}$2 product of the reaction is : ...
+NaIAcetone product; SN2 product of the reaction is :
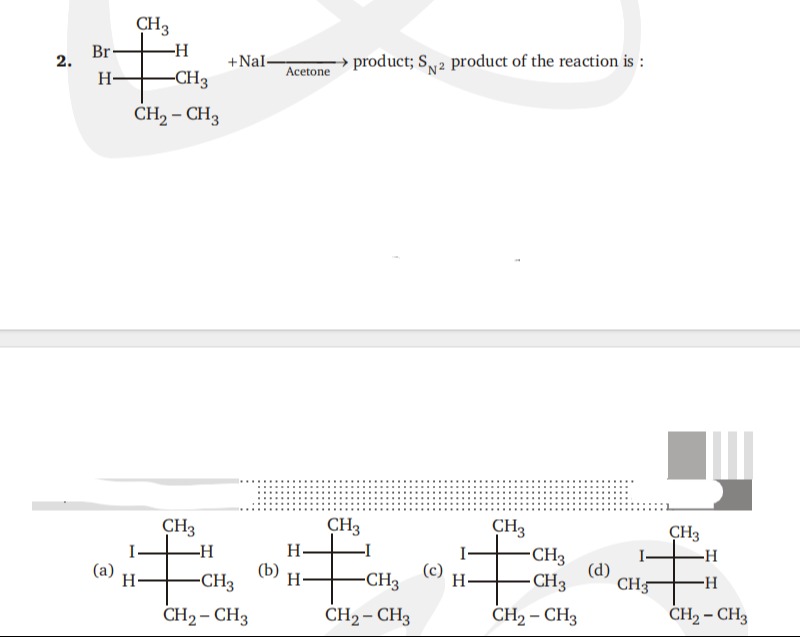
H -CH3 CH2-CH3
H -CH3 CH2-CH3
CH3 -CH3 CH2-CH3
H -H CH2-CH3
(b)
Solution
The given reaction is a Finkelstein reaction, which is an SN2 reaction. SN2 reactions proceed with inversion of configuration at the chiral center where the substitution occurs. The reactant is 2-bromo-3-methylpentane. The substitution occurs at the carbon atom bearing the bromine, which is C2. Therefore, the configuration at C2 will be inverted, while the configuration at C3 will remain unchanged.
First, let's determine the configuration of the chiral centers C2 and C3 in the reactant using the given Fischer projection. The Fischer projection is:
CH3
|
Br --- C2 --- H
|
H --- C3 --- CH3
|
CH2-CH3
For C2, the substituents are Br, H, CH3, and the group attached to C3. Priority order: Br (1), C3 (2), CH3 (3), H (4). To determine the priority between C3 and CH3, we look at the atoms attached to C3 and the carbon of CH3. C3 is bonded to H, CH3, CH2CH3, and C2. The group attached to C2 is -CH(H)(CH3)(CH2CH3). The first atom is C3, which is bonded to H, CH3, CH2CH3. Let's compare the group attached to C2 with CH3. The group attached to C2 is -CH(H)(CH3)(CH2CH3). The carbon atom is bonded to H, C, C. The carbon in CH3 is bonded to H, H, H. So, -CH(H)(CH3)(CH2CH3) has higher priority than CH3. So, the priority order on C2 is: Br (1), -CH(H)(CH3)(CH2CH3) (2), CH3 (3), H (4). In the Fischer projection, H is on the horizontal line. The path 1->2->3 is Br -> C3 -> CH3. This path is clockwise, which normally corresponds to R configuration. Since H is on the horizontal line, we reverse the configuration. So, C2 has S configuration.
For C3, the substituents are H, CH3, CH2CH3, and the group attached to C2. Priority order: C2 (1), CH2CH3 (2), CH3 (3), H (4). To determine the priority of C2, we look at the atoms attached to C2: Br, H, CH3. Comparing C2, CH2CH3, and CH3: C2 is bonded to Br, C, H. CH2CH3: C is bonded to C, H, H. CH3: C is bonded to H, H, H. Comparing the atoms with highest atomic number, C2 has Br, CH2CH3 has C, CH3 has H. So C2 has the highest priority. Comparing CH2CH3 and CH3, the first carbon in CH2CH3 is bonded to C, H, H. The carbon in CH3 is bonded to H, H, H. So CH2CH3 has higher priority than CH3. So, on C3, the priority order is C2 (1), CH2CH3 (2), CH3 (3), H (4). In the Fischer projection, H is on the horizontal line. The path 1->2->3 is C2 -> CH2CH3 -> CH3. This path is clockwise, which normally corresponds to R configuration. Since H is on the horizontal line, we reverse the configuration. So, C3 has S configuration. Thus, the reactant is (2S,3S)-2-bromo-3-methylpentane.
In the SN2 reaction, the substitution of Br by I occurs at C2 with inversion of configuration. The configuration at C3 remains unchanged. So, the product will have R configuration at C2 and S configuration at C3. The product is 2-iodo-3-methylpentane.
Let's determine the configuration of the options. Option (a):
CH3
|
I --- C2 --- H
|
H --- C3 --- CH3
|
CH2-CH3
C2: I (1), C3 (2), CH3 (3), H (4). Path 1->2->3 is I -> C3 -> CH3. This is clockwise. H is horizontal, so reverse. C2 is S. C3: C2 (1), CH2CH3 (2), CH3 (3), H (4). Path 1->2->3 is C2 -> CH2CH3 -> CH3. This is clockwise. H is horizontal, so reverse. C3 is S. Configuration is (2S,3S).
Option (b):
CH3
|
H --- C2 --- I
|
H --- C3 --- CH3
|
CH2-CH3
C2: I (1), C3 (2), CH3 (3), H (4). Path 1->2->3 is I -> C3 -> CH3. This is counterclockwise. H is horizontal, so reverse. C2 is R. C3: C2 (1), CH2CH3 (2), CH3 (3), H (4). Path 1->2->3 is C2 -> CH2CH3 -> CH3. This is clockwise. H is horizontal, so reverse. C3 is S. Configuration is (2R,3S).
Option (c):
CH3
|
I --- C2 --- CH3
|
H --- C3 --- H
|
CH2-CH3
This is not 2-iodo-3-methylpentane.
Option (d):
CH3
|
I --- C2 --- H
|
CH3--- C3 --- H
|
CH2-CH3
This is not a standard Fischer projection. Let's interpret it as a Fischer projection with vertical chain C2-C3. On C2: I (left), H (right), CH3 (top). On C3: CH3 (left), H (right), CH2CH3 (bottom). C2: I (1), C3 (2), CH3 (3), H (4). Path 1->2->3 is I -> C3 -> CH3. This is clockwise. H is horizontal, so reverse. C2 is S. C3: C2 (1), CH2CH3 (2), CH3 (3), H (4). Path 1->2->3 is C2 -> CH2CH3 -> CH3. This is clockwise. H is horizontal, so reverse. C3 is S. Configuration is (2S,3S).
Based on the inversion of configuration at C2, the product should have R configuration at C2 and S configuration at C3. Option (b) has (2R,3S) configuration.
Final check: Reactant: (2S,3S)-2-bromo-3-methylpentane. SN2 reaction at C2. Inversion of configuration at C2. Configuration at C3 remains the same. Product configuration at C2 is R. Product configuration at C3 is S. Product is (2R,3S)-2-iodo-3-methylpentane. Option (b) represents (2R,3S)-2-iodo-3-methylpentane.
