Question
Question: \(CH_3 - CH = CH_2\xrightarrow{Cl_2/H_2O}\) The correct product is: A)  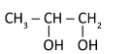
B) 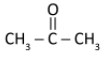
C) 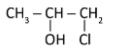
D) 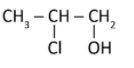
Solution
The given reactant reacts with chlorine in the presence of water, the pi bond will break and the addition of chlorine and hydroxide will take place. The double bond present here is important for the reaction to occur, after reaction of acid and water hypochlorous acid will be formed in which Cl acts as electrophile. To decide the correct answer, we should know the proper mechanism.
Complete answer:
Alkenes react with HOCl the intermediate containing carbocation will be formed, as shown.
Hydroxyl will form the bond with secondary carbocation of the intermediate. As per Markovnikov’s rule the halogen (nucleophile) will go to the carbon with a greater number of alkyl atoms but here hydroxyl will get the preference, because it acts as nucleophile and Cl act as electrophile. So the major product is option (C) and not option (D). Other options are wrong as the mechanism suggests.
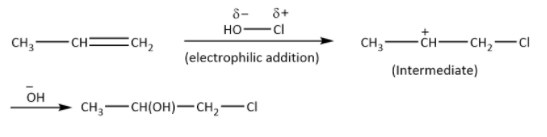
Hence, the correct answer is option (C).
Note: This type of reaction in organic chemistry is called electrophilic addition reaction. Electrophilic addition reactions are characteristic of those compounds that contain multiple bonds such as alkenes and alkynes. The attack of electrophile occurs at the carbon-carbon double or triple bond that results in the formation of a carbocation in the initial step. The carbocation is further attacked by a nucleophile.
