Question
Question: \( CH_2=CH_2+Br_2\xrightarrow{NaCl}\text{ }Products. \) Which of the following products is not forme...
CH2=CH2+Br2NaCl Products. Which of the following products is not formed in the above reaction?
(A) BrCH2CH2Br
(B) ClCH2CH2Cl
(C) BrCH2CH2Cl
(D) All are formed
Solution
Hint : As we know that the alkenes are very reactive towards electrophilic substitution reaction. The reactivity is due to its electrons present in it. All the halides are electronegative in nature and can act as a nucleophile.
Complete Step By Step Answer:
The ethene molecule contains a double bond, one is sigma ( σ ) bonding and the other is pi ( π ) bonding. The bonding electrons are not much stable due to side wise overlapping. Therefore, the pi electrons are responsible for the reactivity of ethene compounds toward electrophilic substitution.
When the ethene attacks on bromine, the atom’s shared pair electrons repel with ethene pi electrons and then one bromine atom splits into bromide ion and one is bromonium ion. This bromonium ion is very electron deficient, the pi electrons on each carbon of ethene are polarized by positive charge on bromine and hence cyclization occurs with the ethene compound.
Now, the free bromide ion acts as nucleophile due to negative charge, attacks on this cyclized product and forms 1,2−dibromoethane , As we can see that there is also sodium chloride which in aqueous medium gives chloride ion. The chloride ion acts as nucleophile due to negative charge and attacks on also cyclized product and form product as 1−bromo−2−chloroethane
Br2→Br−+Br+ and NaClH2OClaq−+Naaq+
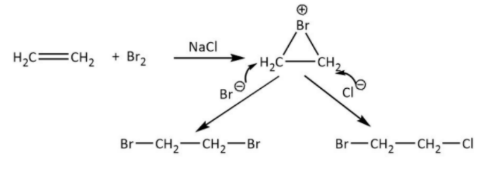
Therefore, the correct answer is option B.
Note :
There are two types of state of electrons; Ground state- in which electrons are paired up. The excited state -in which electrons are in triplet state. The ethene electrons when reacted with bromonium ions are excited to triplet state then the cyclization occurs.
