Question
Question: The system $N_2O_4 \rightleftharpoons 2NO_2$ maintained in a closed vessel at 60°C & a pressure of 5...
The system N2O4⇌2NO2 maintained in a closed vessel at 60°C & a pressure of 5 atm has an average (i.e. observed) molecular weight of 69. At what pressure (in atm) at the same temperature would the observed molecular weight be (230/3)?
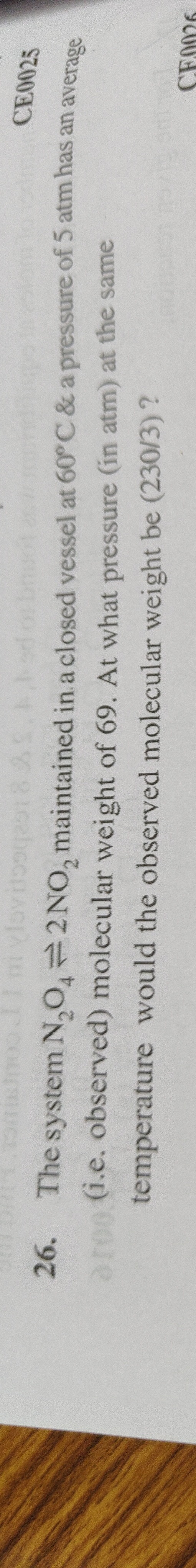
4.5 atm
Solution
For the dissociation N2O4⇌2NO2, the theoretical molecular weight of N2O4 is Mtheory=92 g/mol. The observed molecular weight (Mobs) is related to the degree of dissociation (α) by the formula Mobs=1+(n−1)αMtheory. For this reaction, n=2, so Mobs=1+αMtheory. This implies 1+α=MobsMtheory. In a closed vessel at constant temperature, the pressure (P) is directly proportional to the total number of moles, which is ntotal=n0(1+α). Thus, P∝(1+α). Combining these, we get P2P1=1+α21+α1=Mtheory/Mobs,2Mtheory/Mobs,1=Mobs,1Mobs,2. This simplifies to P1Mobs,1=P2Mobs,2. Using the given values, 5 atm×69 g/mol=P2×3230 g/mol, which yields P2=4.5 atm.
