Question
Question: Cathode rays are streams of fast moving negatively charged particles. Their speed range is (Consider...
Cathode rays are streams of fast moving negatively charged particles. Their speed range is (Consider c=3x108ms−1)
a.0.1c to 0.2c
b.c
c.greater than c
d.Around 10−5c to 10−3c
Solution
By using cathode rays, J.J. Thomson studied the properties of negatively charged particles, electrons. He discovered a specific charge of the electron which is the ratio of charge by mass (me).
We will use a formula from the experiment which J.J. Thomson performed to study electrons.
Formula used:
v=m2eV
Where v is the speed of an electron, e is electric charge (1.6x10−19C), Vis the potential difference, and mis the mass of an electron (9.1x10−31Kg).
Complete step by step answer:
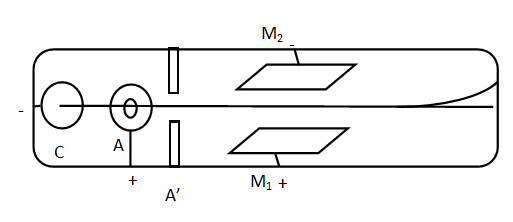
C− cathode, A− anode, A′− metal electrode, M1,M2− metal plates
A very large potential difference V is applied between cathode C and anode A. A beam of electrons passes through anode and metal electrodes A′. The electron beam then passes through metal plates M1 and M2.M1 is connected to the positive terminal of supply and M2, a negative power supply maintaining constant potential difference V. Beams of electron travels through these plates and hits the end of a tube which is coated by fluorescent material, which glows when an electron strikes it. The potential difference V between C and A then the speed of the electron coming out of the anode is given by
21mv2=eV
v=m2eV
If we take V=3000
v=9.1×10−312×1.6×3000×10−19
v=9.19600×10−19+31
v=1054.94×1012
v=32.47×106
cv=332.47×108106
cv=108×10−2
v=0.1c
Similarly, if we increase the potential difference V, the speed of an electron can be increased.
Looking at options b) and c) is not possible and from the above calculation, option d) is also incorrect.
Hence if we look at the options a) 0.1c to 0.2c is correct.
Additional Information:
This theory contributed to a better understanding of the structure of an atom. And the most important discovery Thomson made was that the characteristics of cathode rays or electrons were unaffected by the material of the electrodes or the nature of the gas present in the cathode ray tube. Overall, we can conclude that electrons are the fundamental constituents of all atoms.
Note:
In the cathode ray, if both magnetic field and electric field are applied then both the field will cancel each other out and electrons would pass through the tube undeflected.
While calculating 10 to powers try to solve them separately as calculation becomes easier.
