Question
Question: Carry out the following conversions: I) Phenol to benzoquinone II) Propanone to 2- methyl propan...
Carry out the following conversions:
I) Phenol to benzoquinone
II) Propanone to 2- methyl propan- 2- ol
III) Propene to propan-2-ol
Solution
To answer this question, you must recall the reactions occurring in organic compounds on reacting them with various reagents.
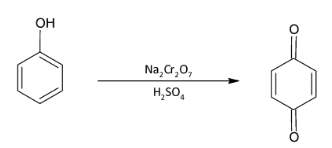
I)Phenol is an alcohol while benzoquinone is a ketone. So the conversion reaction would be an oxidation reaction.
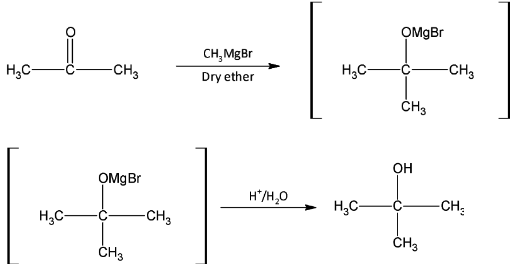
II)Propanone is a ketone and 2- methyl propan- 2- ol is an alcohol. The reaction will be a reduction reaction.
III)The conversion of propene to propan- 2- ol is an additional reaction of water over the double bond of the alkene.
Complete step-by-step answer: I) We know that phenol is an aromatic alcohol while benzoquinone is a quinone present in a single ring. There are two keto groups present in conjugation with the double bonds of the benzene ring. Oxidation is the loss of hydrogen atom or the addition of oxygen atom. Since an extra oxygen atom is also added in the conversion, we need very strong oxidizing agents. So, we react phenol with sodium dichromate in presence of sulphuric acid as they are very strong oxidizing agents. The reaction is given as:
II) Propanone has one less carbon atom than the required product. So we first react propanone with methyl magnesium bromide. Hydrolysis of the organo- metallic compound will gives us the required product 2- methyl propan- 2- ol
III) Propene is converted to propan- 2- ol by addition of water over the double bond in presence of dilute sulphuric acid. The reaction proceeds through Markovnikov’s addition reaction mechanism.
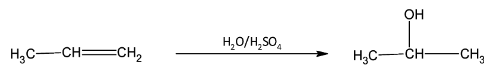
Note: Markovnikov’s rule states that in case of additional reaction of water over alkene a more substituted alcohol is formed. The reaction proceeds in such a way so that a more stable intermediate carbocation is formed.
