Question
Question: Carbon suboxide \({C_3}{O_2}\) has: A: Linear structure B: Bent structure C: Trigonal planar s...
Carbon suboxide C3O2 has:
A: Linear structure
B: Bent structure
C: Trigonal planar structure
D: Distorted tetrahedral structure
Solution
In carbon suboxide three atoms of carbon are linked with two atoms of oxygen. In this compound there are double bonds between the atoms. All the structures given in options are formed when there is no lone pair on the central atom.
Complete step by step answer:
Carbon suboxide is a colorless gas. It has a strong pungent smell. This gas reacts with water to give malonic acid. Carbon suboxide is one of the stable members of the series of linear oxocarbons. Oxo Carbons are represented by the formula O=Cn=O. Chemical formula of carbon suboxide is C3O2. In this molecule there are three atoms of carbon and two atoms of oxygen. Structure of this compound is as follows:
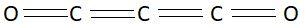
In this picture we can clearly see that carbon suboxide has linear structure.
So, the correct answer is Option A .
Additional Information:
Carbon suboxide is a foul smelling gas which is produced by the dehydration of malonic acid, CH2(COOH)2, with P4O10 in vacuum. At 25∘C this compound polymerises to a highly color solid substance. Under the influence of ultraviolet light this compound decomposes to ketene. Ketene is a highly reactive compound. Carbon suboxide is an acid anhydride of malonic acid. Carbon suboxide reacts slowly with water to give malonic acid. Carbon suboxide has a bent structure in gaseous phase.
Note:
Carbon dioxide is also a molecule of carbon and oxygen. Chemical formula of this molecule is CO2. Like carbon suboxide, carbon dioxide also has linear structure. Hybridization of this molecule is sp. In this molecule there is no lone pair on the central atom.
