Question
Question: Carbon monoxide is carried around a closed abc in which bc is an isothermal process, as shown in fig...
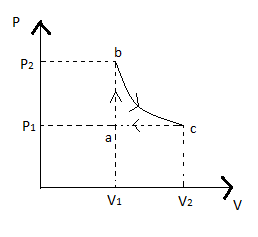
Carbon monoxide is carried around a closed abc in which bc is an isothermal process, as shown in fig. The gas absorbs 7000J of heat as its temperature increases from 300 K to 1000 K in going from a to b. The quantity of heat rejected by the gas during the process CA Is
A. 4200 J
B. 5000 J
C. 9000 J
D. 9800 J
Solution
In this question, the PV cycle is given where curve bc going through the isothermal process, ab is going through the isochoric process since volume is constant, and ca be going through the isobaric process as pressure is constant.
Complete step by step answer:
Given in cycle abc, bc is an isothermal process
In cycle, we can see ab is an isochoric process since volume is constant where 7000J of heat is absorbed as its temperature increases from 300 K to 1000 K
Hence isochoric process for AB will be
ΔUAB=ΔQAB−ΔWAB−−(i)
Where
ΔWAB=0, since the area under the curve, a to b is zero
ΔQAB=7000Jis given
Hence we can write equation (i) as
Now for the isochoric process, we know
ΔU=nCvΔT−−(iii)
Hence we can write equation (iii) for the curve AB as
WhereΔUAB=7000J, from equation (ii), hence we get
7000=n25R(1000−300) n25R700=7000 nR=520 nR=4−−(iv)Now for curve ac, since the pressure is constant, so the process is isobaric, this is given as
ΔUAC=nCpΔT−−(v)
Since the curve bc is isothermal hence we can say temperature at b will be equal to the temperature at c; hence we can write equation (v) as
Where the value of nR=4from equation (iv), hence we can write
ΔUAC=−nR27700 =−4×27×700 =−9800JSo the quantity of heat rejected by the gas during the process ca is =9800J
Option D is correct.
Note: The process in which there is no change in the pressure or the process in which the pressure remains constant is known as Isobaric Process.
The process in which there is no change in the volume or the process in which the volume remains constant is known as Isochoric Process.
