Question
Question: Carbon donor ligands are strong ligands and usually form low spin complexes. Among the complexes giv...
Carbon donor ligands are strong ligands and usually form low spin complexes. Among the complexes given below, select the high spin complexes.
A.)[Fe(CN)6]3−
B.)[Fe(CN)6]4−
C.)[Fe(C2O4)3]3−
D.) None of these
Solution
High spin complexes are those which have more spin than that possessed by the parent metal. CN− are strong field ligands as compared to C2O42−. In CN−, the electrons mostly pair up while in the case of C2O42−- , the pairing of electrons is comparatively less.
Complete answer:
Carbon donor ligands usually form low spin complexes, which means that the spin of the resulting complex will be less than that of the parent metal.
Among the given options, [Fe(C2O4)3]3− is the only high spin complex, it is because the ligand C2O42−is a weak field donor ligand as compared to CN− ligand.
In the case of CN− ligands, they pair up as mostly they form inner ‘d’ orbital complexes.
The electrons in C2O42− do not pair up as they do in CN- ligands.
In the case of high spin complexes, their spin magnetic moment increases which is given by the formula :
spinonlymagneticmoment=n(n+2)
Where ‘n’ is the number of unpaired electrons.
So, the correct answer is “Option C”.
Additional Information:
Now, we can look at how to determine the high spin and low spin complexes:
(i) By determining the shape of the comlex .For Eg: tetrahedral, octahedral shape etc.
(ii) By determining the oxidation state of the central metal atom . For example : In [Fe(CN)6]3−, the oxidation state of central metal atom Fe is +3.
(iii) By determining the ‘d’ electron configuration of the central metal atom . For Eg: in[Fe(CN)6]3−, the hybridization is d2sp3 , that means the complex forms an inner ‘d’ orbital complex.
(iii) By drawing the crystal field diagram of the complex with respect to the geometry of the complex.
(iv) By determining whether the splitting energy is greater than the pairing energy.
(v) By determining the strength of the ligand (i.e.by using the help of spectrochemical series)
Crystal field theory (CFT):
Proposed by Bethe and Vavleck.
CFT considers metal- ligand bond as ionic bond, metal acts as the positive point charge and ligands as the negative point charge.
When the ligands approach the central metal, the degenerate ‘d’ orbitals of the metal may undergo splitting , which is called crystal field splitting.
The splitting pattern depends on geometry of the element.
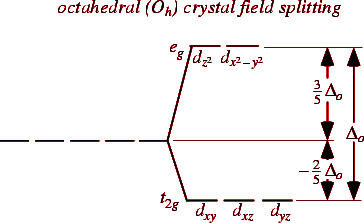
Note:
Crystal field splitting is not a perfect theory. It also has limitations. For Eg: The CFT considers metal-ligand bond as ionic bond. But the theory depended on geometry which is a contradiction.
