Question
Question: Carbocation which is most stable...
Carbocation which is most stable
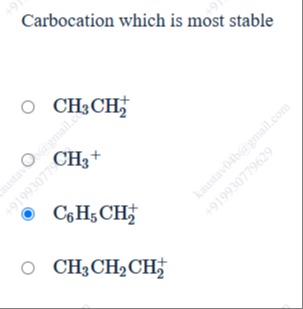
CH3CH2+
CH3+
C6H5CH2+
CH3CH2CH2+
C6H5CH2+
Solution
Carbocation stability is enhanced by resonance, hyperconjugation, and inductive effects. Resonance stabilization is the most significant factor. The benzyl carbocation (C6H5CH2+) is stabilized by resonance with the pi electrons of the phenyl ring, which delocalizes the positive charge. The other carbocations (methyl, ethyl, n-propyl) are stabilized mainly by hyperconjugation and inductive effects, which are less effective than resonance. The methyl carbocation has no significant stabilization. Ethyl (3 alpha H) and n-propyl (2 alpha H) are primary carbocations stabilized by hyperconjugation. Resonance stabilization makes the benzyl carbocation the most stable among the given options.
