Question
Question: Cannizzaro’s reaction is not given by : A.
B.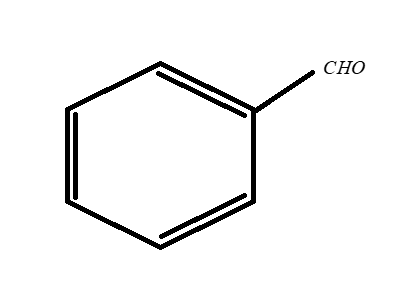
C.HCHO
D.CH3CHO
Solution
Firstly in the question we need to get the explanation of what is cannizzaro reaction and the reaction mechanism behind it. The reaction in which non-enolizable aldehyde given a molecule of primary alcohol and one carboxylic acid. Now we have to define the mechanism of the reaction. Then we can give the two conditions which don't allow the reaction ( existence of enol form and 3−α hydrogen attached to the carbonyl group). Then we can see which option doesn't follow it and get the answer.
Complete step by step answer:
Cannizzaro reaction is a characteristic reaction for the carboxylic acids which don’t have an enol form. It is a chemical reaction in which the given base-induced disproportionation of two molecules of a non-enolizable aldehyde takes place in order to give a primary alcohol and a carboxylic acid.
The given reaction mechanism of the Cannizzaro reaction is as follows:
The Cannizzaro reaction is a reaction that involves a nucleophilic acyl substitution, which is done on an aldehyde. In this process the leaving group concurrently attacks the other aldehyde in the second step of mechanism. In the first step the hydroxide attacks a carbonyl.
There are mainly two conditions when the Cannizzaro reaction does not takes place :
When the enol form exists and is attainable.
Secondly when the carboxyl group is directly attached to a carbon with 3−α hydrogen atoms.
In the given question among all the questions only the acetaldehyde doesn’t fulfill the conditions.
Therefore, the right answer is option D, CH3CHO .
Note: The Cannizzaro reaction, named after its discoverer Stanislao Cannizzaro who gave it in the year 1853. He got it when he was reacting the benzaldehyde with the potash. He obtained benzyl alcohol and the potassium benzoate and thus named the reaction after him.
