Question
Question: Cannizzaro reaction will be given by 
A.(i), (ii) and (iii)
B. (i) and (ii)
C.(ii) and (iii)
D. (i) and (iv)
Solution
In the Cannizzaro reaction two aldehyde molecules undergo disproportionation. The aldehyde molecules that cannot undergo keto-enol tautomerism give the Cannizzaro reaction. Cannizzaro reaction takes place in the alkali solution.
Complete step by step answer:
In the Cannizzaro reaction, two aldehyde molecules react in presence of a base and form a carboxylic acid and alcohol.
The base attacks the carbonyl group of one alcohol molecule and forms a tetrahedral anion.
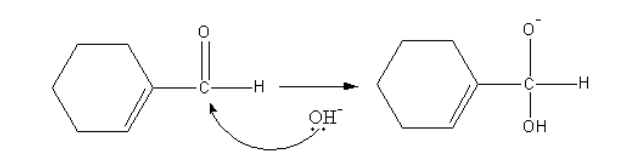
The tetrahedral anion forms acid and removes a hydride. This hydride attacks the carbonyl group of another aldehyde molecule.
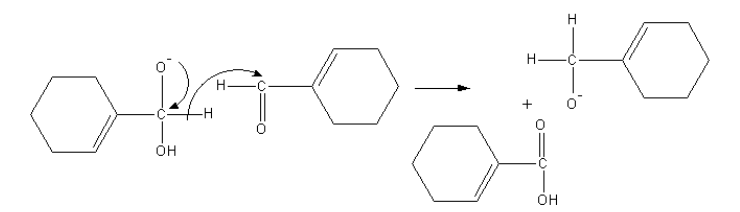
Another anion abstract proton from acid and form alcohol and acid anion get proton from solvent water, so an acid and alcohol form.
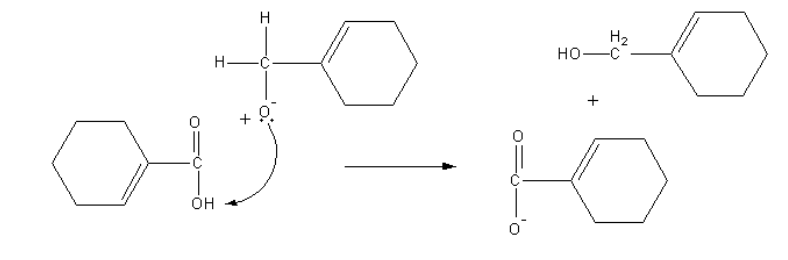
In the Cannizzaro reaction, nucleophilic attacks take place on aldehyde, so the aldehyde having no alpha-hydrogen gives Cannizzaro reaction.
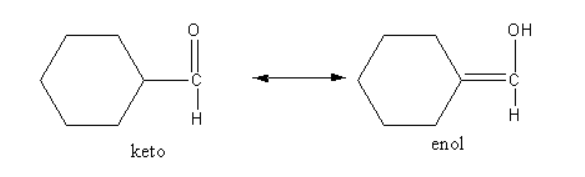
If the aldehyde has alpha-hydrogen, the aldehyde undergoes keto-enol tautomerism which decreases the electrophilicity of the carbonyl group, so the nucleophilic attacks decrease.
The keto-enol tautomerism is shown as follows:
Compound (i), cyclohexane carbaldehyde has alpha hydrogen, so it does not give Cannizzaro reaction.
Compound (ii), cyclohex−1−ene carbaldehyde has no alpha hydrogen, so it gives Cannizzaro reaction.
Compound (iii), benzaldehyde has no alpha hydrogen, so it gives Cannizzaro reaction.
Compound (iv) acetaldehyde has alpha hydrogen, so it does not give Cannizzaro reaction.
So, compound (ii) and (iii) give a Cannizzaro reaction.
**Therefore, option (C) (ii) and (iii) is correct.
Note: **
The aldehyde having alpha-hydrogen can undergo keto-enol tautomerism. The double-bonded carbon in keto form is more electrophilic than the carbon bonded with the hydroxyl group in enol form. The alpha-hydrogen having aldehydes gives an aldol condensation reaction. The products of aldol condensation reaction are beta-hydroxy ketone and beta-hydroxy aldehyde.
