Question
Question: Calculate the ‘x’ in \(M{n^{ + x}}\) if \(\mu \) = 3.87 BM:- A. 2 B. 3 C. 4 D. 5...
Calculate the ‘x’ in Mn+x if μ = 3.87 BM:-
A. 2
B. 3
C. 4
D. 5
Solution
given this question we have magnetic moment(μ)=3.87. So in the first stage we will calculate the number of electrons required to get 3.87 BM amount of magnetic moment. After that we will make electronic configuration of manganese. After that step we will again set the charge of ions on the manganese.
Complete answer:
We all know that the formula for the magnetic moment is: n(n+2) , where n=number of unpaired electrons in the configuration of the atom or ion.
Mathematically, μ=n(n+2)-(I)
Now putting the value of μ in the given equation.
So,$
3.87 = \sqrt {n(n + 2)} \\
Nowsquaringthebothsideintheaboveequation:
15 = n(n + 2) \\
{n^2} + 2n - 15 = 0 \\
Onsolvingthisquadraticequation:Weget,
{n^2} + 5n - 3n - 15 = 0 \\
n(n + 5) - 3(n + 5) = 0 \\
n = 3,n = - 5
\therefore nisthenumberofunpairedelectronsintheionsoitcannotbeanegativenumberbecausethenumberofelectronscan‘tbenegative.Hence,n=3Nowwehavegotthenumberofelectronsintheionis3.NowwewillmakeelectronicconfigurationofmanganeseAsweallknowthatatomicnumberofmanganeseis25Soelectronicconfiguration,Mn(25)=1{s^2},2{s^2},2{p^6},3{s^2},3{p^6},4{s^2},3{d^5}Nowwehavetoarrangetheelectronicconfigurationofmanganesesuchthatitsconfigurationhave3unpairedelectronsinthevalenceshellSo,wehavetorelease4electronsfromthemanganesetoget3unpairedelectronsinthevalenceshellTherefore,electronicconfigurationwillbe:M{n^{ + 4}}(21) = 1{s^2},2{s^2},2{p^6},3{s^2},3{p^{^6}},3{d^3}$
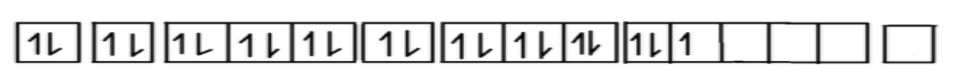
Now we can see in the configuration we have 3 electrons in the valence shell of manganese ions.
So value of x will be 4 in the given question.
**Therefore, the correct answer will be option number C.
Note:**
Magnetic moment is the property of a magnet that spreads the magnetic field to attract the material it provides mechanical moment to attract materials. Magnetic moment is also called that it is the strength of a magnet by which it attracts the other materials.
