Question
Question: Calculate the work done by adiabatic compression of one mole of an ideal gas (mono atomic) from an i...
Calculate the work done by adiabatic compression of one mole of an ideal gas (mono atomic) from an initial pressure of 1 atm to final pressure of 2 atm. Initial temperature = 300 K.
(a)If the process is carried out reversibly
(b) If the process is carried out irreversibly against 2 atm external pressure.
Compute the final volume reached by gas in two cases and describe the work graphically.
Solution
Adiabatic compression is taking place, means that the energy is transferred to the surroundings. Here compression is taking place that causes a temperature rise in the gas.
Formula used: Wadiabatic=∫−PdV
Complete answer:
(a) We have been given 1 mole of an ideal gas with initial pressure P1 = 1 atm, final pressureP2= 2 atm. With initial temperature= 300 K. Heat capacity of an ideal gas at constant volume Cv= 3 23R , so, r= 35
Now to calculate work done , when the process is carried reversibly, we have to take out the volume,
As, PV=nRT
We have, P = P2−P1= 1 , n = 1, R= 0.082, T= 300 K
So, 1×V=1×0.082×300
V1 = 24.63
For reversible process in adiabatic compression, P1V1=P2V2
1×(24.63)r=2×V2rwhere r = 1.66, so, (V224.6)1.66
Therefore, 1.66×log(V224.6)=log2
So, V2=18.15L
Now, in adiabatic compression work done is, Wadiabatic=∫−PdV
Wadiabatic=∫V1−rCdV= 1194.72 J
So, work done is 1194.72 J
Now, for final temperature,P1−rVr = constant for reversible compression,
P21−35T235=P1−32T13
2−2300[300T]5=1
300T=451
T = 41/5× 300
T2 = 395.85
Hence, work done in this case is 1194.72 J and final volume is V2=18.15L
(b) now, we have to calculate work done when the process is irreversible against 2 atm external pressure.
For this, PV=nRT = 300 R
We know that, nCvΔT=−P2(V2−V1)
1×23R(T2−T1)=−2(V2−V1)
23R(T2−300)=−V2×R+2×300R
23R−450=−T2.1+600
25T2=1050
T2=52100
T2=420K
So, volume will be, V2=2R×420 = 210 R
V2= 17.24 L
So, work done will be W=−2(V2−V1)
W=−2(210R−300R)
W = 180 R
W = 1496.525 J
Hence, work done in this case is 1496.525 J and final volume is V2= 17.24 L
For both the volumes the graph between pressure and volume will be plotted as,
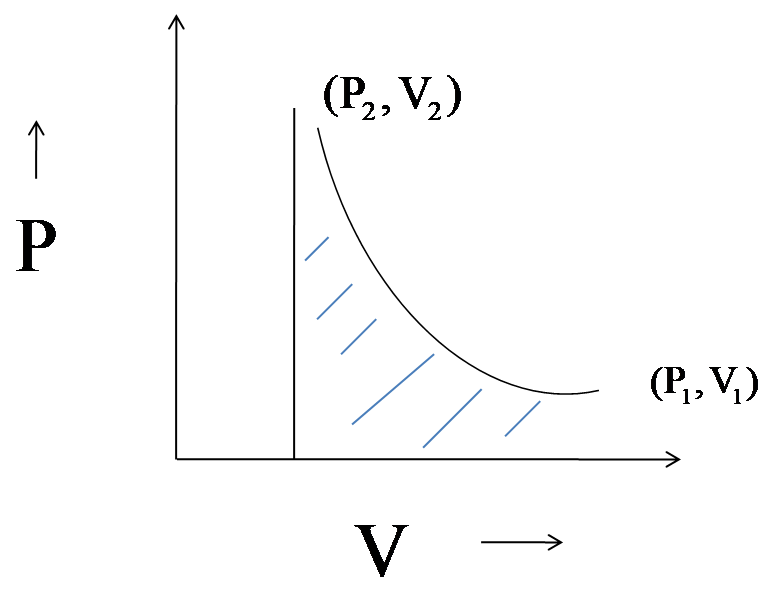
The work done in adiabatic compression is the area under the curve. In both the cases, the final volume is greater than the initial volume.
Note:
In adiabatic compression we will obtain the final temperatures in both the cases to be greater than initial temperatures, as the gas has been compressed. In the second case, the final temperature is higher than the first case, because external pressure of 2 atm is also applied.
