Question
Question: Calculate the total number of unpaired electrons in \[{\left[ {Cr{{\left( {{H_2}O} \right)}_6}} \rig...
Calculate the total number of unpaired electrons in [Cr(H2O)6]3+?
Solution
We have to remember the VBT (Valence Bond Theory), it is useful for explaining the chemical bonding. VBT is based on covalent interactions between the central metal and the ligands. This theory is given by Linus Pauling. This theory also explains the geometry, magnetic behavior and the formation of a complex compound. The name of the compound [Cr(H2O)6]3+ is hexaaquachromium(III) ion.
Complete step by step answer:
Let us see how the Valence Bond Theory explains the bonding in [Cr(H2O)6]3+, hexaaquachromium(III) ion.
First of all we know that chromium has an atomic number of 24 and outer most electronic configuration is 3d54s1, because the half-filled d orbitals have extra stability. So, in chromium, one electron of 4s orbital goes to 3d orbital and it is extra stable. Hexaaquachromium (III) ion coordination number is six, the total number of electron pairs accepted by the central metal atom (chromium) will be equal to six.
The outer most electronic configuration of chromium is 3d54s1,
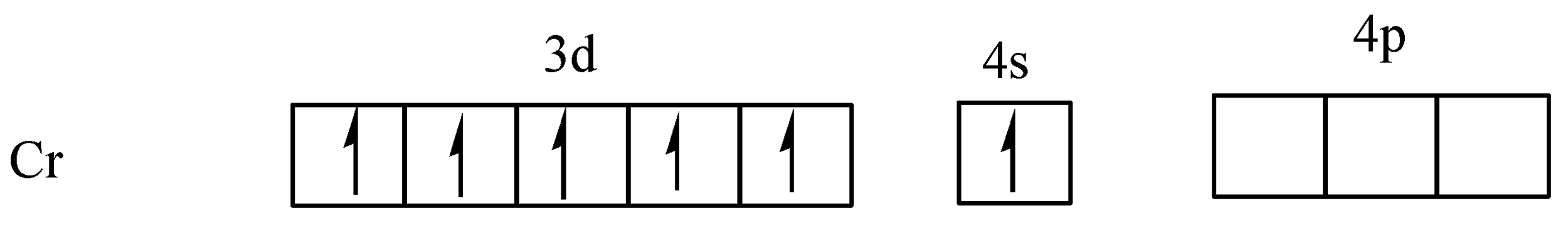
From the above diagram clearly shows that the five orbitals are singly filled. In the complex,[Cr(H2O)6]3+, the ligand H2O is a weak ligand, so no electron pair takes place and chromium has +3 oxidation state,
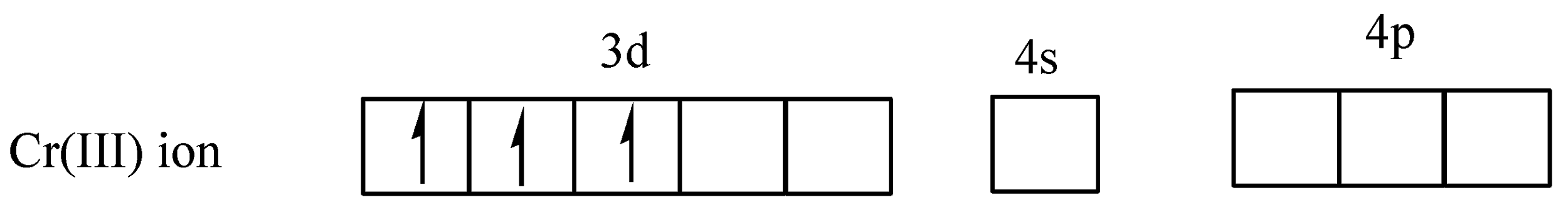
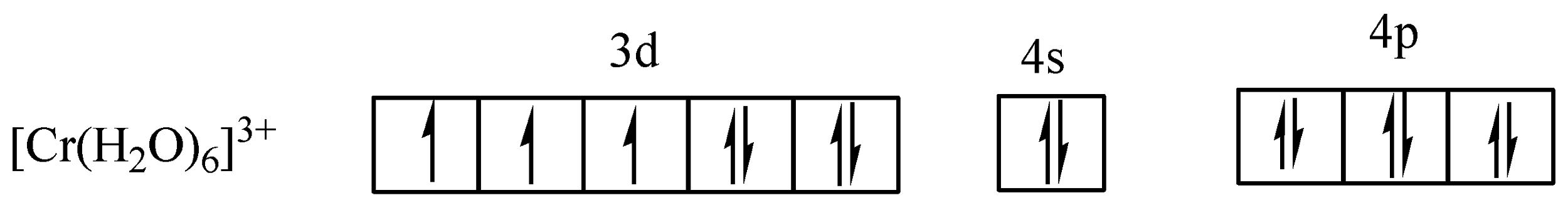
In (c) the arrows (↑↓) represent electron pairs donated by H2O.
In (c) the six water molecules forming d2sp3 hybridization occur resulting in an octahedral of the complex and indicated the complex is paramagnetic.
From (c), there are three (3) unpaired electrons in a hexaaquachromium(III) ion ([Cr(H2O)6]3+) complex.
Note: We have to remember that the valence Bond Theory important aspect is the condition of maximum overlap, which leads to the formation of the strongest possible bonds. Some of the ligands are strong ligands (e.g. CN−,NO2−,SCN−etc.), thus forced pairing will occur and the electrons are paired.
