Question
Question: Calculate the packing efficiency of a simple cubic cell?...
Calculate the packing efficiency of a simple cubic cell?
Solution
The packing efficiency can be defined as the ratio of the volume of a single atom in a unit cell to the volume of the entire unit cell multiplied by 100 . It is a measure of the amount of volume that is occupied in a unit cell.
FORMULA USED: PE=a334πr3×100
Where r is the radius of the atom and a is the edge length of the simple cubic cell.
Complete answer:
The cubic unit cell is the simplest form of packing seen in a simple cubic structure.The packing efficiency of the simple cubic lattice can be found based on the length of the edge. The edge length can be derived as follows:
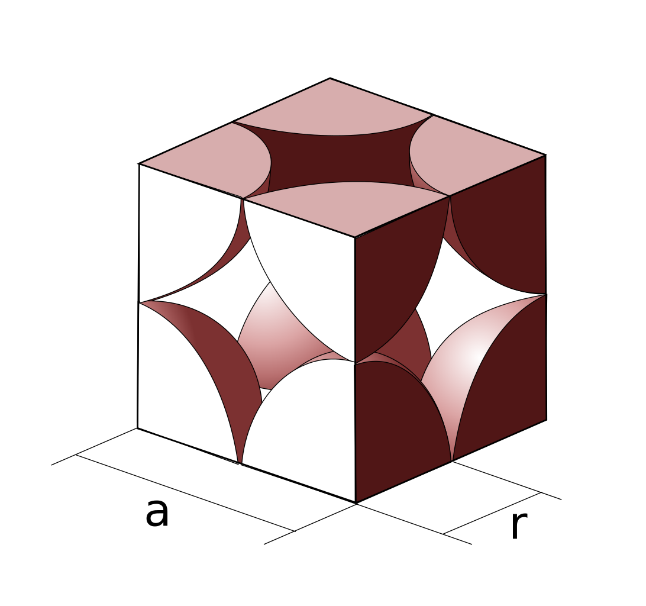
First, we have to consider the following image: we can see that the halves of two atoms lie along the edge of the cube. This means that if we consider the radius of each atom to be r we can then concur that the length of the edge will be represented as follows,
a=2r
Here a is the edge length of the simple cubic lattice.
Using the formula mentioned above it will now be easy to derive the packing efficiency of this lattice. This is demonstrated below:
PE= a334πr3 ×100
we have to plug the value for edge length.
⇒(2r)334πr3×100
Since r is present both in the numerator and denominator, we can cancel that term to get the equation given below.
⇒(2)334π×100
⇒3×84π×100
cancelling the multiples of 4 in the given equation, we now get,
⇒2×3π×100
⇒0.523×100
After multiplying with 100 we get the percentage which is,
⇒52.3%
Therefore, the packing efficiency of the simple cubic lattice is 52.3% .
Note: The 52.3% packing efficiency means that out of the 100% of space or volume that is present in the lattice only 52.3% of the lattice is occupied by the atoms and the remaining 47.7% is made up of empty space also known as voids or interstitial spaces.
It is also imperative to remember the edge length of different crystal lattices as they differ as in face centered, body centered and others.
