Question
Question: Calculate the number of molecules so Sulphur (\({{S}_{8}}\)) present in 16g of solid Sulphur....
Calculate the number of molecules so Sulphur (S8) present in 16g of solid Sulphur.
Solution
Using the Avogadro’s law which states 1 mol of gas at NTP or STP contains Avogadro number of particles i.e. 6.023×1023 and occupies 22.4 L of the gas, we can easily find the number of molecules of the Sulphur atom in 16g as we know that 256 g of Sulphur (S8) contains 6.023×1023 molecules. Now find the molecules of Sulphur.
Complete step by step answer:
Sulphur is a non-metal and lies in the 16th group of the period table and comes under the category of the oxygen family and belongs to the p-block elements. It has an atomic number as 16 and mass number as 32.
It is pale yellow and crystalline solid and has no taste but has little characteristic smell. It is insoluble in water and its particles float on the surface of the water and form a yellow film on the surface of the water.
In S8 molecule , there are eight Sulphur molecules which are covalently bonded to each other and forms a closed crown shaped structure as:
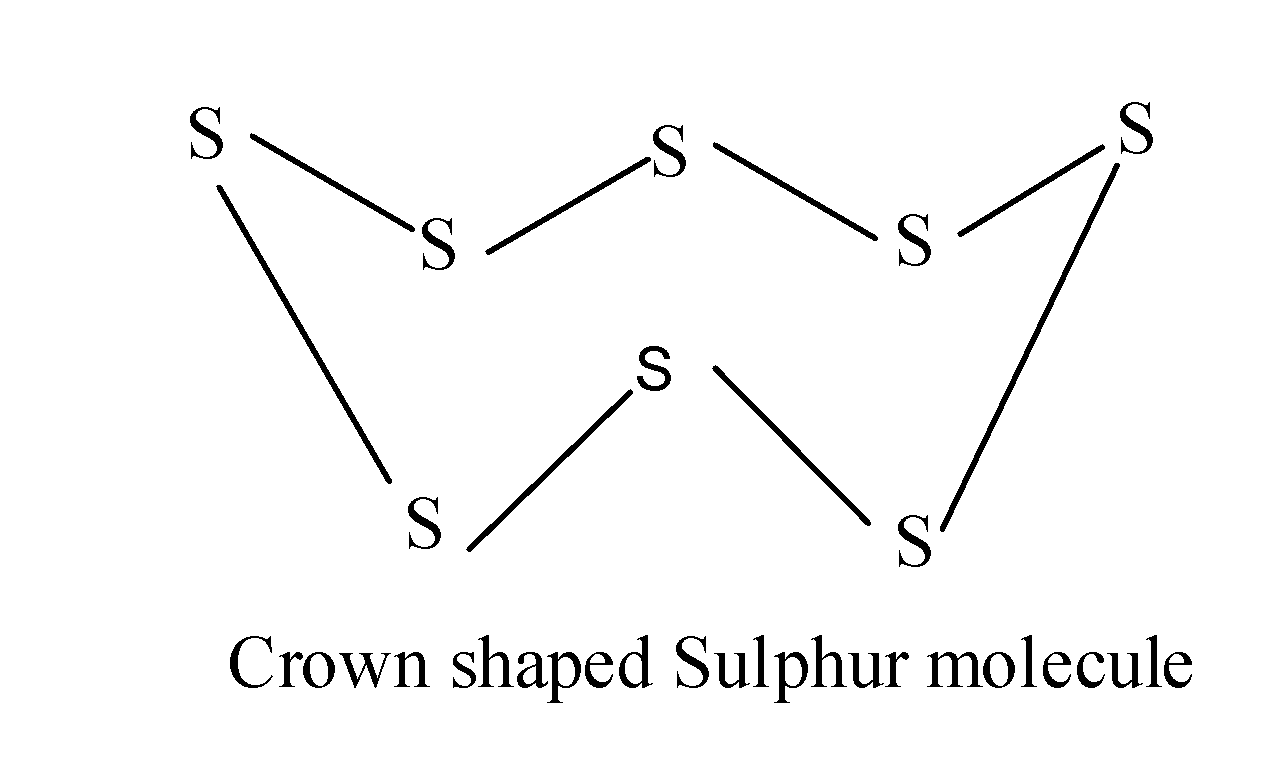
Since, we know the molecular mass of one Sulphur atom is 32.
And in S8, there are 8 Sulphur atoms, so the mass molecular mass of solid Sulphur in the crown shaped structure is = 8×32 =256 g
Now, the given numerical is based on the Avogadro’s law which states that any 1 mole of gas at the standard conditions of temperature(i.e. ∘C) and pressure(i.e. 1 atm) contains Avogadro number of particles i.e. 6.023×1023 and occupies the volume of 22.4L at STP or NTP
So, from this we can say that;
256 g of Sulphur at STP contains = 6.023×1023 molecules
1 g of Sulphur at STP contains = 2566.023×1023 molecules
16 g of Sulphur at STP contains = 2566.023×1023×16 molecules
=3.76×1022 molecules.
Hence, the number of molecules of Sulphur(S8) present in 16g of solid Sulphur is 3.76×1022.
Note: Avogadro’s law is applicable only at the conditions of standard temperature and pressure and if the pressure and temperature are constant , then on increasing the amount of the gas, its volume also increases and vice-versa.
