Question
Question: Calculate the number of atoms per unit cell of FCC and BCC crystal structure....
Calculate the number of atoms per unit cell of FCC and BCC crystal structure.
Solution
Hint: Solve the given question using basic knowledge of solid state systems. Solving the question by using diagrams of unit cells would be beneficial.
Complete step by step answer:
This is the structure of a simple or primitive cubic unit cell-
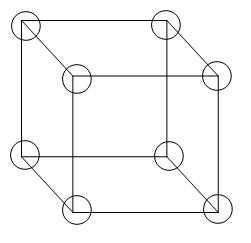
From the diagram we can see that 8 atoms are arranged in all the corners of the cube, therefore, the contribution per unit cell will be 1 atom,
∵8x81=1 atom or molecule
This is the structure of a body-centred unit cell-
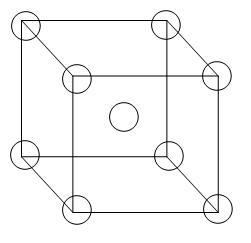
From the diagram we can see that 8 atoms are arranged in all the corners of the cube and there is 1 atom in the centre of the unit cell. Therefore, the contribution per unit cell will be –
= 1 atom (primitive) + 1 atom (body centred)
=2
This is the structure of a face-centred unit cell-
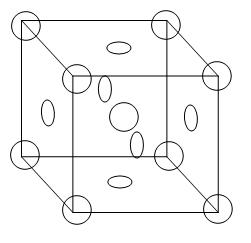
From the diagram we can see that 8 atoms are arranged in all the corners of the cube and there is 1 atom in the centre of the unit cell. In addition to this, there are atoms on each face of the cube. Therefore, the contribution of atoms on the cube = 6x21=3
Therefore, total contribution per unit cell will be –
= 1 atom (primitive) + 1 atom (body centred) + 3 (face centred)
= 4.
Therefore, the answer is –
The number of atoms per unit cell of FCC crystal structure = 4 and,
The number of atoms per unit cell of BCC crystal structure = 2.
Note: Unit cell is the simplest unit of a complete crystal lattice. It is the most uniform unit used for analysis of the structure.
