Question
Question: Calculate the mass of Ag deposited at cathode when 96.5 coulomb of electricity is passed through a s...
Calculate the mass of Ag deposited at cathode when 96.5 coulomb of electricity is passed through a solution of AgNO3.
(Molar Mass of Ag = 108 g mol−1)
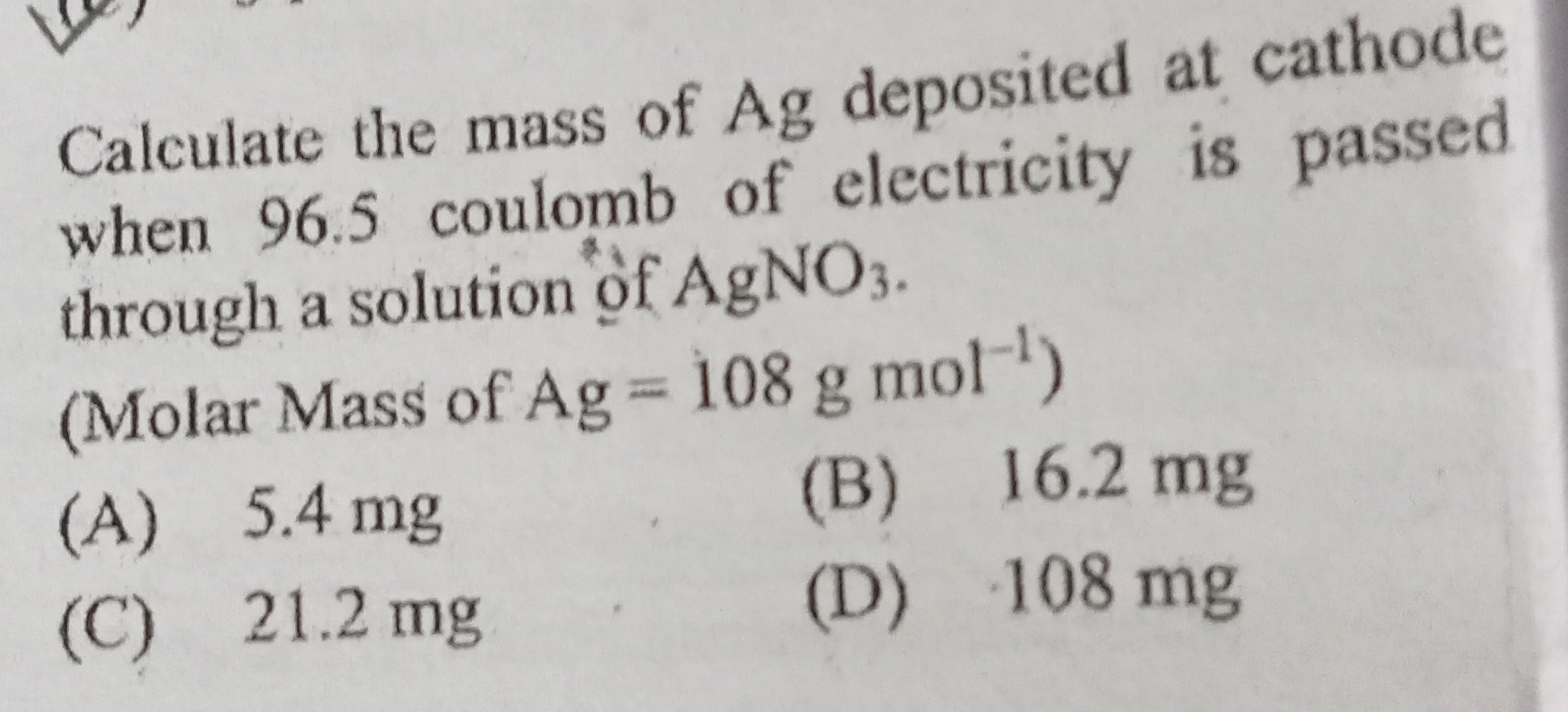
A
5.4 mg
B
16.2 mg
C
21.2 mg
D
108 mg
Answer
108 mg
Explanation
Solution
The deposition of silver occurs via the reaction:
Ag++e−→Ag
Using Faraday's law:
Massdeposited=FMolarmass×Q
Here,
Molar mass of Ag = 108 g/mol, Q = 96.5 C, F = 96500 C/mol.
So,
Mass=96500108×96.5=9650010422=0.108g=108mg
